Mapping the dynamics and nanoscale organization of synaptic adhesion proteins using monomeric streptavidin - PubMed (original) (raw)
Mapping the dynamics and nanoscale organization of synaptic adhesion proteins using monomeric streptavidin
Ingrid Chamma et al. Nat Commun. 2016.
Abstract
The advent of super-resolution imaging (SRI) has created a need for optimized labelling strategies. We present a new method relying on fluorophore-conjugated monomeric streptavidin (mSA) to label membrane proteins carrying a short, enzymatically biotinylated tag, compatible with SRI techniques including uPAINT, STED and dSTORM. We demonstrate efficient and specific labelling of target proteins in confined intercellular and organotypic tissues, with reduced steric hindrance and no crosslinking compared with multivalent probes. We use mSA to decipher the dynamics and nanoscale organization of the synaptic adhesion molecules neurexin-1β, neuroligin-1 (Nlg1) and leucine-rich-repeat transmembrane protein 2 (LRRTM2) in a dual-colour configuration with GFP nanobody, and show that these proteins are diffusionally trapped at synapses where they form apposed trans-synaptic adhesive structures. Furthermore, Nlg1 is dynamic, disperse and sensitive to synaptic stimulation, whereas LRRTM2 is organized in compact and stable nanodomains. Thus, mSA is a versatile tool to image membrane proteins at high resolution in complex live environments, providing novel information about the nano-organization of biological structures.
Figures
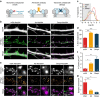
Figure 1. Super-resolution imaging of AP-Nlg1 with mSA or biotin antibody or tetrameric streptavidin.
(a) Schematic diagram of AP-Nlg1 labelled with three different probes (mSA, monoclonal biotin antibody or streptavidin), conjugated to Atto 594 for uPAINT or Alexa 647 for dSTORM. (b) Examples of DIV 7 neurons co-expressing EGFP as a volume marker, AP-Nlg1 and BirAER, and labelled as described above. Middle panels display AP-Nlg1 trajectories calculated from stacks of 4,000 images with 20-ms exposure time (green: fast-diffusing pool, that is, _D_>0.01 μm2 s−1; magenta: slow diffusing pool, that is, D<0.01 μm2 s−1). Merged images show EGFP (grey) overlaid with AP-Nlg1 trajectories. Note that mSA explores larger surface areas with faster diffusion than multivalent ligands. (c) Distribution of AP-Nlg1 diffusion coefficients in a semi-log plot, where the grey-shaded area represents slow trajectories (that is, with D<0.01 μm2 s−1). (d) Corresponding percentage of slow trajectories measured in the three different conditions (***P<0.0001). Data are from three different experiments. (e) Examples of DIV 15 neurons co-expressing Homer1c-GFP, AP-Nlg1 and BirAER, and labelled with the three Alexa647-conjugated probes shown in a. Top panels: Homer1c-GFP signal showing mature synapses (white). Middle panels: super-resolved AP-Nlg1 detection maps generated from 40,000 frames with 20-ms integration time. Bottom panels: merged images showing AP-Nlg1 detections (green) overlaid with Homer1c-GFP (magenta). Note the presence of large AP-Nlg1 aggregates in anti-biotin and streptavidin-labelled neurons. (f) Histogram showing the number of Nlg1 clusters per μm2 in the three conditions (**P<0.01). (g) Histogram showing the synaptic enrichment of AP-Nlg1 compared with the shaft (**P<0.01, *P<0.05). Data are from two different experiments. Numbers in the bar charts represent the number of cells examined.
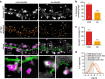
Figure 2. Different ability of mSA and biotin antibody to label AP-Nlg1 within the synaptic cleft in live conditions.
(a) DIV 15 neurons expressing Homer1c-GFP, AP-Nlg1 and BirAER were labelled with mSA or anti-biotin conjugated to Atto594 to track individual AP-Nlg1 molecules by uPAINT. From top to bottom: Homer1c-GFP signal staining mature synapses; super-resolved AP-Nlg1 detection maps; merged images showing extrasynaptic AP-Nlg1 trajectories (green) and synaptic trajectories (magenta) overlaid with Homer1c-GFP signals (grey); insets show that mSA-stained Nlg1 fills the entire synaptic area, whereas antibiotin remains on the edge of the postsynaptic density. (b) Percentage of synapses containing AP-Nlg1 labelled with mSA or biotin antibody (***P<0.0001). (c) Percentage of the synaptic area occupied by AP-Nlg1 when labelled with mSA or anti-biotin (**P<0.01). (d) Semi-log distribution of synaptic (solid lines) and extrasynaptic (dashed lines) diffusion coefficients for AP-Nlg1 measured with the two different probes. Data are from two different experiments. Numbers in the bar charts represent the number of cells analysed.
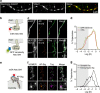
Figure 3. Super-resolution imaging of AP-Nlg1 in organotypic hippocampal slices.
(a) Confocal (EGFP, left), STED (mSA-Atto 647N, middle) and merged (right) images acquired from live neurons expressing GFP, AP-Nlg1 and BirAER in organotypic hippocampal slices. Images are projections of a z-stack of 60 planes taken by 1-μm increments and colour-coded with respect to sample depth. (b) Linescan measurements of mSA-Atto 647N and GFP staining along the z axis. The reduction in mSA-Atto 647N intensity with the sample depth likely reflects reduced laser penetration rather than weaker staining, since the GFP signal also decreases with depth. (c) High-magnification deconvoluted STED projection of mSA-labelled Nlg1 in a hippocampal slice shows the accumulation of Nlg1 at dendritic spines. (d) Linescans of GFP and mSA-Atto 647N fluorescence intensity in the shaft membrane and in a dendritic spine normalized to the respective fluorescence of GFP and Atto 647N in the shaft. (e) Wide field image of mSA-Alexa647 selectively labelling one neuron expressing GFP, AP-Nlg1 and BirAER, a few microns deep from the surface of an organotypic brain slice. (f) Astigmatic-based 3D dSTORM imaging in an organotypic brain slice from Nlg1 KO mice: (i) wide field image of mSA-Alexa647-labelled AP-Nlg1. (ii) 3D dSTORM-reconstructed image of a dendritic segment based on 1,038,506 single-molecule localizations from 64,000 images. The image is colour-coded with respect to the z distance (−600 to +600 nm). (iii) Normalized localization detection maps integrated within z=±400 nm. (iv) Magnified view of iii showing the enrichment of Nlg1 in dendritic spines.
Figure 4. Comparison of mSA and GFP nanobody.
(a) DIV 15 axons expressing GFP-Nrx1β were live-labelled using anti-GFP nanobody-Atto647, followed by immunolabelling for VGlut1 to stain pre-synaptic glutamatergic terminals. The merged image shows the colocalization between GFP-Nrx1β (red) and VGlut1 (green). (b) Schematics showing the labelling of AP-Nrx1β with mSA-Atto594 (top) and GFP-Nrx1β with GFP nanobody-Atto594 (bottom). (c) Examples of axonal regions from DIV 15 neurons expressing EGFP, AP-Nrx1β and BirAER (top); or GFP-Nrx1β (bottom). From left to right: GFP signal, Nrx1 β detection maps, colour-coded Nrx1β trajectory maps (green: fast-diffusing pool, that is, _D_>0.01 μm2 s−1; magenta: slow diffusing pool, that is, D<0.01 μm2 s−1), merged image showing Nrx1β trajectories overlaid with GFP (grey). (d) Distributions of the diffusion coefficients obtained for AP-Nrx1β or GFP-Nrx1β (mSA, _n_=6; GFP nanobody, _n_=5 cells from two different experiments). (e) Schematics showing the structure of the AMPA receptor auxiliary protein stargazin (Stg) with the insertion of an AP tag in the first extracellular protein loop. (f) Example of DIV 15 neurons co-expressing the synaptic marker Homer1c-GFP, AP-Stg and BirAER. AP-Stg individual molecules were tracked using mSA-Atto594 by uPAINT. Super-resolved AP-Stg localization and trajectory maps were reconstructed from 4,000 images of 20-ms exposure time, with the same colour code as in b. (g) Diffusion coefficient distribution of AP-Stg inside and outside synapses (_n_=6 cells from two different experiments).
Figure 5. Dual-colour super-resolution imaging of _trans_-synaptic contacts between neurexin-1β and neuroligin-1.
Neurons co-expressing AP-Nlg1, BirAER and Homer1c-GFP or expressing BFP-Nrx1β were co-cultured for 15 days and labelled with mSA-Atto 594 and Atto 647N nanobody. (a) Schematics of labelled adhesion molecules at the synapse. (b) Integrated density of Nlg1 (red) and Nrx1β (green) molecules at axon/dendrite contacts, identified from Homer1c-GFP signal (grey). (c) Percentages of slow trajectories for Nlg1 (red) and Nrx1β (green) were measured inside (solid) and outside (stippled) synapses (Nrx1β, _n_=12; Nlg1, _n_=10 cells from three different experiments ***P<0.0001). (d) Number of Nrx1β (green), Nlg1 (red) and apposed Nrx1β/Nlg1 (yellow) synaptic clusters before and after 10 min EGTA treatment (_n_=6 cells for each condition from two different experiments). (e) Examples of synaptic Nlg1 and Nrx1β clusters and corresponding two-dimensional anisotropic Gaussian fits. (f) Frequency distributions of Nlg1 and Nrx1β cluster lengths (Nlg1: median 80.44, interquartile range (IQR) 56–102 nm, 531 clusters from 14 cells; Nrx1β: median 75.17, IQR of 62–109 nm, 235 clusters from 11 cells. Data are from three different experiments). (g) Destabilization of _trans_-synaptic Nrx1β/Nlg1 contacts by NMDA application (20 μM, 10 min). (h) Number of Nlg1 and Nrx1β trajectories over time on a 10-min NMDA treatment (red, green) or in control condition (black). The trajectory counts were normalized by their respective numbers before treatment (_n_=6 cells for each condition from three different experiments ***P<0.0001, one-way analysis of variance).
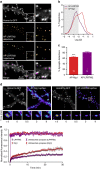
Figure 6. Comparison of Nlg1 and LRRTM2 dynamics in mature hippocampal neurons.
(a) DIV 15 neurons expressing AP-LRRTM2, Homer1c-GFP and BirAER were labelled using mSA-Atto 594 for uPAINT imaging of single LRRTM2 molecules. Super-resolved localization and trajectory maps are shown (green, fast-diffusing molecules, magenta, slow-moving molecules). Note the absence of diffusion on dendritic shafts. (b) Semi-log distribution of AP-LRRTM2 and AP-Nlg1 diffusion coefficients. (c) Corresponding percentage of synaptic LRRTM2 and Nlg1 detections by uPAINT (LRRTM2, _n_=8; Nlg1, _n_=15 cells from three different experiments). (d) FRAP experiments performed on AP-Nlg1 and AP-LRRTM2 labelled with mSA-Atto594. (e) Corresponding normalized fluorescence recovery curves. The intensity of unbleached synapses is shown as control for observational photobleaching. Solid lines represent fits of the mean data points with the diffusion-reaction equation given in the Methods. The parameters obtained for Nlg1 and LRRTM2 were the fraction of free molecules _φ_=0.27 and 0.19 and the turnover rate of adhesions _k_reac=1.4 × 10−2 and 5.0 × 10−3 min−1, respectively. The ratio of all synaptic molecules versus free molecules (1/φ) gives 3.5 for Nlg1 and 5.1 for LRRTM2, closely corresponding to the synaptic enrichment values measured by dSTORM (Nlg1, _n_=25; LRRTM2, _n_=18; Nlg1 unbleached, _n_=10; LRRTM2 unbleached, _n_=8 cells for each condition from three different experiments).
Figure 7. Comparison of Nlg1 and LRRTM2 organization in mature hippocampal neurons.
(a,b) DIV 15 neurons expressing either AP-Nlg1 or AP-LRRTM2, plus Homer1c-GFP and BirAER were labelled at high density with mSA-Alexa 647 for dSTORM imaging. Integrated densities over 40,000 frames are shown. (c,d) AP-Nlg1 and LRRTM2 fluorescence across linescans represented in a,b insets show local fluorescence accumulation within synapses. (e) Dispersion of AP-Nlg1 and AP-LRRTM2 molecules within synapses represented as the distribution of distances from individual synaptic detections relatively to the centroid of the Homer1c-GFP signal (LRRTM2, _n_=7; Nlg1, _n_=5). (f) Distribution of the number of locally enriched AP-Nlg1 and AP-LRRTM2 domains within synapses (LRRTM2, _n_=7; Nlg1, _n_=5). (g) The median sizes of AP-Nlg1 and AP-LRRTM2 locally enriched synaptic domains (Nlg1, 87.00, IQR 68–110, _n_=5; LRRTM2, 98.35, IQR 76–122, _n_=7; ***P<0.0001). (h) Synaptic area in DIV 15 neurons electroporated with AP-Nlg1 or AP-LRRTM2 based on the Homer1c-GFP signal. (i) Representative STORM image of AP-Nlg1r expressed on a knockdown background in DIV 15 rat neurons, shown with the corresponding low-resolution mSA-Atto647 labelling. (j) Average intensity corresponding to the linescan in i showing local AP-Nlg1r fluorescence accumulation within a spine in a knockdown background, similar to AP-Nlg1. (k) Synaptic enrichment of AP-Nlg1, AP-Nlg1r co-expressed on a knockdown background and AP-LRRTM2 with respect to shaft levels (AP-Nlg1, 2.73±0.62, _n_=5; AP-Nlg1r, 2.54±0.34, _n_=4; AP-LRRTM2, 5.29±0.81 _n_=7; *P<0.05). Data are from three different experiments for AP-Nlg1 and AP-LRRTM2, and two experiments for AP-Nlg1r.
Similar articles
- Activity-dependent proteolytic cleavage of neuroligin-1.
Suzuki K, Hayashi Y, Nakahara S, Kumazaki H, Prox J, Horiuchi K, Zeng M, Tanimura S, Nishiyama Y, Osawa S, Sehara-Fujisawa A, Saftig P, Yokoshima S, Fukuyama T, Matsuki N, Koyama R, Tomita T, Iwatsubo T. Suzuki K, et al. Neuron. 2012 Oct 18;76(2):410-22. doi: 10.1016/j.neuron.2012.10.003. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083742 - Crystal Structure of an Engineered LRRTM2 Synaptic Adhesion Molecule and a Model for Neurexin Binding.
Paatero A, Rosti K, Shkumatov AV, Sele C, Brunello C, Kysenius K, Singha P, Jokinen V, Huttunen H, Kajander T. Paatero A, et al. Biochemistry. 2016 Feb 16;55(6):914-26. doi: 10.1021/acs.biochem.5b00971. Epub 2016 Feb 3. Biochemistry. 2016. PMID: 26785044 - Nanoscale organization of synaptic adhesion proteins revealed by single-molecule localization microscopy.
Chamma I, Levet F, Sibarita JB, Sainlos M, Thoumine O. Chamma I, et al. Neurophotonics. 2016 Oct;3(4):041810. doi: 10.1117/1.NPh.3.4.041810. Epub 2016 Nov 3. Neurophotonics. 2016. PMID: 27872870 Free PMC article. - Dynamics, nanoscale organization, and function of synaptic adhesion molecules.
Chamma I, Thoumine O. Chamma I, et al. Mol Cell Neurosci. 2018 Sep;91:95-107. doi: 10.1016/j.mcn.2018.04.007. Epub 2018 Apr 17. Mol Cell Neurosci. 2018. PMID: 29673914 Review. - The SALM/Lrfn family of leucine-rich repeat-containing cell adhesion molecules.
Nam J, Mah W, Kim E. Nam J, et al. Semin Cell Dev Biol. 2011 Jul;22(5):492-8. doi: 10.1016/j.semcdb.2011.06.005. Epub 2011 Jun 29. Semin Cell Dev Biol. 2011. PMID: 21736948 Review.
Cited by
- CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation.
Hosokawa T, Liu PW, Cai Q, Ferreira JS, Levet F, Butler C, Sibarita JB, Choquet D, Groc L, Hosy E, Zhang M, Hayashi Y. Hosokawa T, et al. Nat Neurosci. 2021 Jun;24(6):777-785. doi: 10.1038/s41593-021-00843-3. Epub 2021 Apr 29. Nat Neurosci. 2021. PMID: 33927400 - High-Resolution Fluorescence Imaging Combined With Computer Simulations to Quantitate Surface Dynamics and Nanoscale Organization of Neuroligin-1 at Synapses.
Lagardère M, Drouet A, Sainlos M, Thoumine O. Lagardère M, et al. Front Synaptic Neurosci. 2022 Apr 25;14:835427. doi: 10.3389/fnsyn.2022.835427. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35546899 Free PMC article. - Neurexin-3 subsynaptic densities are spatially distinct from Neurexin-1 and essential for excitatory synapse nanoscale organization in the hippocampus.
Lloyd BA, Han Y, Roth R, Zhang B, Aoto J. Lloyd BA, et al. Nat Commun. 2023 Aug 5;14(1):4706. doi: 10.1038/s41467-023-40419-2. Nat Commun. 2023. PMID: 37543682 Free PMC article. - Synthetic mimics of biotin/(strept)avidin.
Liu W, Samanta SK, Smith BD, Isaacs L. Liu W, et al. Chem Soc Rev. 2017 May 9;46(9):2391-2403. doi: 10.1039/c7cs00011a. Chem Soc Rev. 2017. PMID: 28191579 Free PMC article. Review. - Concerted roles of LRRTM1 and SynCAM 1 in organizing prefrontal cortex synapses and cognitive functions.
de Arce KP, Ribic A, Chowdhury D, Watters K, Thompson GJ, Sanganahalli BG, Lippard ETC, Rohlmann A, Strittmatter SM, Missler M, Hyder F, Biederer T. de Arce KP, et al. Nat Commun. 2023 Jan 28;14(1):459. doi: 10.1038/s41467-023-36042-w. Nat Commun. 2023. PMID: 36709330 Free PMC article.
References
- Triller A. & Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron 59, 359–374 (2008). - PubMed
- Willig K. I. et al.. Nanoscale resolution in GFP-based microscopy. Nat. Methods 3, 721–723 (2006). - PubMed
- Manley S. et al.. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials