Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA - PubMed (original) (raw)
doi: 10.1038/ncomms11122.
Johannes Kettunen 1 2 3 4, Peter Würtz 1, Harmen H M Draisma 7 8 9, Toomas Haller 10, Rajesh Rawal 11 12, Anika Vaarhorst 13, Antti J Kangas 1, Leo-Pekka Lyytikäinen 14, Matti Pirinen 15, René Pool 7 8, Antti-Pekka Sarin 2 15, Pasi Soininen 1 3, Taru Tukiainen 16 17, Qin Wang 1 3, Mika Tiainen 1 3, Tuulia Tynkkynen 1 3, Najaf Amin 6, Tanja Zeller 18 19, Marian Beekman 13, Joris Deelen 13, Ko Willems van Dijk 5 20, Tõnu Esko 10, Jouke-Jan Hottenga 7 8, Elisabeth M van Leeuwen 6, Terho Lehtimäki 14, Evelin Mihailov 10, Richard J Rose 21 22, Anton J M de Craen 23, Christian Gieger 11 12, Mika Kähönen 24, Markus Perola 2 10 15, Stefan Blankenberg 18 19, Markku J Savolainen 4 25, Aswin Verhoeven 26, Jorma Viikari 27, Gonneke Willemsen 7 8, Dorret I Boomsma 7 8, Cornelia M van Duijn 6, Johan Eriksson 2 28 29, Antti Jula 2, Marjo-Riitta Järvelin 4 30 31 32, Jaakko Kaprio 15 21 33, Andres Metspalu 10, Olli Raitakari 34 35, Veikko Salomaa 2, P Eline Slagboom 13, Melanie Waldenberger 11 12, Samuli Ripatti 2 15 21 36, Mika Ala-Korpela 1 3 4 37 38 39
Affiliations
- PMID: 27005778
- PMCID: PMC4814583
- DOI: 10.1038/ncomms11122
Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA
Johannes Kettunen et al. Nat Commun. 2016.
Abstract
Genome-wide association studies have identified numerous loci linked with complex diseases, for which the molecular mechanisms remain largely unclear. Comprehensive molecular profiling of circulating metabolites captures highly heritable traits, which can help to uncover metabolic pathophysiology underlying established disease variants. We conduct an extended genome-wide association study of genetic influences on 123 circulating metabolic traits quantified by nuclear magnetic resonance metabolomics from up to 24,925 individuals and identify eight novel loci for amino acids, pyruvate and fatty acids. The LPA locus link with cardiovascular risk exemplifies how detailed metabolic profiling may inform underlying aetiology via extensive associations with very-low-density lipoprotein and triglyceride metabolism. Genetic fine mapping and Mendelian randomization uncover wide-spread causal effects of lipoprotein(a) on overall lipoprotein metabolism and we assess potential pleiotropic consequences of genetically elevated lipoprotein(a) on diverse morbidities via electronic health-care records. Our findings strengthen the argument for safe LPA-targeted intervention to reduce cardiovascular risk.
Conflict of interest statement
S.B. has received honoraria from Abbott Diagnostics, SIEMENS, Thermo Fisher and Roche Diagnostics and is a consultant for Thermo Fisher. The sponsor played no role in the design or conduct of this study; in the management, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript or in the decision to submit the manuscript for publication. P.W., A.J.K., P.S. and M.A.-K. are shareholders of Brainshake Ltd. (), a company offering NMR-based metabolite profiling. Jo.K., P.W., A.J.K., P.S., Q.W., M.T. and Tu.T. report employment and consulting for Brainshake Ltd. The remaining authors declare no competing financial interests.
Figures
Figure 1. A genome-wide association study for circulating metabolites.
Study was conducted to elucidate the genetic variation of systemic metabolism and to discover new metabolic associations in established loci. We also revealed an intriguing novel relation between Lp(a) and systemic triglyceride and VLDL metabolism. Thereby, we highlighted the _LPA_locus and generated the best possible Lp(a) genetic risk score (GRSLp(a)) that enabled us to clarify causal associations between Lp(a) and systemic triglyceride and lipoprotein metabolism. Further, with the aid of extensive electronic health-care records, we were able to use the GRSLp(a) to show that Lp(a) is associated with ischaemic heart disease but not strongly with other morbidities. Put together, these findings suggest safe molecular intervention on LPA to reduce individual cardiovascular risk.
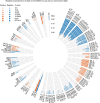
Figure 2. The association pattern of the Lp(a) variant rs10455872 G-allele across all circulating metabolic traits.
Each bar represents the association with respective metabolic trait, the size of the bar is the linear regression effect estimate, colouring refers to effect direction and significance is indicated with filled circles for_P_<2.27 × 10−9 and unfilled circles for P<5 × 10−8. Metabolite abbreviations and sample sizes are given in Supplementary Table 1, the strongest association was observed for the mean diameter of very-low-density lipoprotein particles (VLDL.D).
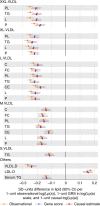
Figure 3. Evaluation of the causative role of the Lp(a) on the circulating metabolic measures via Mendelian randomization.
Yellow linear regression estimates are observational associations, blue are GRSLp(a) estimates and red are the causal effect estimates. Those metabolic traits are listed for which the associations in the meta-analysis were significant with genome-wide threshold (P<2.3 × 10−9). Metabolite abbreviations are given in Supplementary Table 1.
References
- Suhre K. & Gieger C. Genetic variation in metabolic phenotypes: study designs and applications. Nat. Rev. Genet. 13, 759–769 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous