Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome - PubMed (original) (raw)
. 2016 Jun;101(6):707-16.
doi: 10.3324/haematol.2015.137711. Epub 2016 Mar 24.
Markéta Žaliová 1, Martina Suková 2, Marcin Wlodarski 3, Aleš Janda 3, Eva Froňková 4, Vít Campr 5, Kateřina Lejhancová 6, Ondřej Zapletal 7, Dagmar Pospíšilová 8, Zdeňka Černá 9, Tomáš Kuhn 10, Peter Švec 11, Vendula Pelková 1, Zuzana Zemanová 12, Gitte Kerndrup 13, Marry van den Heuvel-Eibrink 14, Vincent van der Velden 15, Charlotte Niemeyer 3, Tomáš Kalina 1, Jan Trka 4, Jan Starý 2, Ondřej Hrušák 4, Ester Mejstříková 16
Affiliations
- PMID: 27013649
- PMCID: PMC5013954
- DOI: 10.3324/haematol.2015.137711
Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome
Michaela Nováková et al. Haematologica. 2016 Jun.
Abstract
GATA-2 deficiency was recently described as common cause of overlapping syndromes of immunodeficiency, lymphedema, familiar myelodysplastic syndrome or acute myeloid leukemia. The aim of our study was to analyze bone marrow and peripheral blood samples of children with myelodysplastic syndrome or aplastic anemia to define prevalence of the GATA2 mutation and to assess whether mutations in GATA-2 transcription factor exhibit specific immunophenotypic features. The prevalence of a GATA2 mutation in a consecutively diagnosed cohort of children was 14% in advanced forms of myelodysplastic syndrome (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and myelodysplasia-related acute myeloid leukemia), 17% in refractory cytopenia of childhood, and 0% in aplastic anemia. In GATA-2-deficient cases, we found the most profound B-cell lymphopenia, including its progenitors in blood and bone marrow, which correlated with significantly diminished intronRSS-Kde recombination excision circles in comparison to other myelodysplastic syndrome/aplastic anemia cases. The other typical features of GATA-2 deficiency (monocytopenia and natural killer cell lymphopenia) were less discriminative. In conclusion, we suggest screening for GATA2 mutations in pediatric myelodysplastic syndrome, preferentially in patients with impaired B-cell homeostasis in bone marrow and peripheral blood (low number of progenitors, intronRSS-Kde recombination excision circles and naïve cells).
Copyright© Ferrata Storti Foundation.
Figures
Figure 1.
Cell populations in bone marrow (BM). (A) B-cell subpopulations and kappa-deleting recombination excision circles (KRECs). (B) T-cell subpopulations and T-cell receptor excision circles (TRECs). (C) Natural killer cells. (D) Monocytes. (E) Progenitors. Braces indicate significant difference between the parameters using the non-parametric Mann-Whitney test (P<0.05). Black lines represent medians. A gray area indicates the range of control samples.
Figure 2.
Cell populations in peripheral blood (PB). (A) B cells and kappa-deleting recombination excision circles (KRECs). (B) T cells and T-cell receptor excision circles (TRECs). (C) Natural killer cells. (D) Monocytes. Braces indicate significant differences between parameters using the non-parametric Mann-Whitney test (P<0.05). A gray area indicates the range of control samples. Absolute counts are shown. In relative counts, similar results were found except for relative T cells, which were increased in GATA-2-deficient patients compared to controls.
Figure 3.
Follow-up peripheral blood (PB) samples of GATA-2-deficient patients. Each column represents one patient. Black lines represent medians. A gray area indicates a normal range for the age category of most of the patients [either 12–18 years for B cells, natural killer (NK) cells; >15 years for monocytes and neutrophils; or >16 years for class switched memory B cells and naïve B cells]. (A) B cells and B-cell subpopulations. (B) Monocytes, NK cells, neutrophils.
Figure 4.
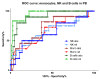
Receiver operating characteristic curves for peripheral blood monocytes, B cells and natural killer (NK) cells in GATA-2-deficient patients in comparison with all other patients with aplastic anemia and myelodysplastic syndromes. Light green: relative B-cell count; dark green: absolute B-cell count; light red: relative monocyte count; dark red: absolute monocyte count; light blue: relative NK-cell count; dark blue: absolute NK-cell count.
Similar articles
- Bone marrow immunophenotyping by flow cytometry in refractory cytopenia of childhood.
Aalbers AM, van den Heuvel-Eibrink MM, Baumann I, Dworzak M, Hasle H, Locatelli F, De Moerloose B, Schmugge M, Mejstrikova E, Nováková M, Zecca M, Zwaan CM, Te Marvelde JG, Langerak AW, van Dongen JJ, Pieters R, Niemeyer CM, van der Velden VH. Aalbers AM, et al. Haematologica. 2015 Mar;100(3):315-23. doi: 10.3324/haematol.2014.107706. Epub 2014 Nov 25. Haematologica. 2015. PMID: 25425683 Free PMC article. Clinical Trial. - Characteristics of the phenotypic abnormalities of bone marrow cells in childhood myelodysplastic syndromes and juvenile myelomonocytic leukemia.
Oliveira AF, Tansini A, Vidal DO, Lopes LF, Metze K, Lorand-Metze I. Oliveira AF, et al. Pediatr Blood Cancer. 2017 Apr;64(4). doi: 10.1002/pbc.26285. Epub 2016 Oct 17. Pediatr Blood Cancer. 2017. PMID: 27748021 - Loss of c-Kit and bone marrow failure upon conditional removal of the GATA-2 C-terminal zinc finger domain in adult mice.
Li HS, Jin J, Liang X, Matatall KA, Ma Y, Zhang H, Ullrich SE, King KY, Sun SC, Watowich SS. Li HS, et al. Eur J Haematol. 2016 Sep;97(3):261-70. doi: 10.1111/ejh.12719. Epub 2016 Jan 14. Eur J Haematol. 2016. PMID: 26660446 Free PMC article. - Germline GATA2 Mutation and Bone Marrow Failure.
McReynolds LJ, Calvo KR, Holland SM. McReynolds LJ, et al. Hematol Oncol Clin North Am. 2018 Aug;32(4):713-728. doi: 10.1016/j.hoc.2018.04.004. Epub 2018 May 28. Hematol Oncol Clin North Am. 2018. PMID: 30047422 Free PMC article. Review. - Pitfalls in the diagnosis of childhood leukaemia.
Chessells JM; haemostasis and thrombosis task force, British committee for standards in haematology. Chessells JM, et al. Br J Haematol. 2001 Sep;114(3):506-11. doi: 10.1046/j.1365-2141.2001.02994.x. Br J Haematol. 2001. PMID: 11552974 Review. No abstract available.
Cited by
- GATA2 deficiency and related myeloid neoplasms.
Wlodarski MW, Collin M, Horwitz MS. Wlodarski MW, et al. Semin Hematol. 2017 Apr;54(2):81-86. doi: 10.1053/j.seminhematol.2017.05.002. Epub 2017 May 10. Semin Hematol. 2017. PMID: 28637621 Free PMC article. - MDS-associated mutations in germline GATA2 mutated patients with hematologic manifestations.
McReynolds LJ, Yang Y, Yuen Wong H, Tang J, Zhang Y, Mulé MP, Daub J, Palmer C, Foruraghi L, Liu Q, Zhu J, Wang W, West RR, Yohe ME, Hsu AP, Hickstein DD, Townsley DM, Holland SM, Calvo KR, Hourigan CS. McReynolds LJ, et al. Leuk Res. 2019 Jan;76:70-75. doi: 10.1016/j.leukres.2018.11.013. Epub 2018 Dec 4. Leuk Res. 2019. PMID: 30578959 Free PMC article. - ASXL1 and STAG2 are common mutations in GATA2 deficiency patients with bone marrow disease and myelodysplastic syndrome.
West RR, Calvo KR, Embree LJ, Wang W, Tuschong LM, Bauer TR, Tillo D, Lack J, Droll S, Hsu AP, Holland SM, Hickstein DD. West RR, et al. Blood Adv. 2022 Feb 8;6(3):793-807. doi: 10.1182/bloodadvances.2021005065. Blood Adv. 2022. PMID: 34529785 Free PMC article. - GATA2 deficiency syndrome: A decade of discovery.
Homan CC, Venugopal P, Arts P, Shahrin NH, Feurstein S, Rawlings L, Lawrence DM, Andrews J, King-Smith SL, Harvey NL, Brown AL, Scott HS, Hahn CN. Homan CC, et al. Hum Mutat. 2021 Nov;42(11):1399-1421. doi: 10.1002/humu.24271. Epub 2021 Aug 31. Hum Mutat. 2021. PMID: 34387894 Free PMC article. - Development of MDS in Pediatric Patients with GATA2 Deficiency: Increased Histone Trimethylation and Deregulated Apoptosis as Potential Drivers of Transformation.
Schreiber F, Piontek G, Schneider-Kimoto Y, Schwarz-Furlan S, De Vito R, Locatelli F, Gengler C, Yoshimi A, Jung A, Klauschen F, Niemeyer CM, Erlacher M, Rudelius M. Schreiber F, et al. Cancers (Basel). 2023 Nov 26;15(23):5594. doi: 10.3390/cancers15235594. Cancers (Basel). 2023. PMID: 38067298 Free PMC article.
References
- Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR. A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada. Br J Haematol. 1999;106(4):1027–1032. - PubMed
- Baumann I, Niemeyer CM, Bennett JM, Shannon K. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency of Research on Cancer (IARC); 2008. p 104–107.
- Baumann I, Führer M, Behrendt S, et al. Morphological differentiation of severe aplastic anaemia from hypocellular refractory cytopenia of childhood: reproducibility of histopathological diagnostic criteria. Histopathology. 2012;61(1):10–17. - PubMed
- Strahm B, Nöllke P, Zecca M, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25(3):455–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical