Role of astrocytic glutamate transporter in alcohol use disorder - PubMed (original) (raw)
Review
Role of astrocytic glutamate transporter in alcohol use disorder
Jennifer R Ayers-Ringler et al. World J Psychiatry. 2016.
Abstract
Alcohol use disorder (AUD) is one of the most widespread neuropsychiatric conditions, having a significant health and socioeconomic impact. According to the 2014 World Health Organization global status report on alcohol and health, the harmful use of alcohol is responsible for 5.9% of all deaths worldwide. Additionally, 5.1% of the global burden of disease and injury is ascribed to alcohol (measured in disability adjusted life years, or disability adjusted life years). Although the neurobiological basis of AUD is highly complex, the corticostriatal circuit contributes significantly to the development of addictive behaviors. In-depth investigation into the changes of the neurotransmitters in this circuit, dopamine, gamma-aminobutyricacid, and glutamate, and their corresponding neuronal receptors in AUD and other addictions enable us to understand the molecular basis of AUD. However, these discoveries have also revealed a dearth of knowledge regarding contributions from non-neuronal sources. Astrocytes, though intimately involved in synaptic function, had until recently been noticeably overlooked in their potential role in AUD. One major function of the astrocyte is protecting neurons from excitotoxicity by removing glutamate from the synapse via excitatory amino acid transporter type 2. The importance of this key transporter in addiction, as well as ethanol withdrawal, has recently become evident, though its regulation is still under investigation. Historically, pharmacotherapy for AUD has been focused on altering the activity of neuronal glutamate receptors. However, recent clinical evidence has supported the animal-based findings, showing that regulating glutamate homeostasis contributes to successful management of recovery from AUD.
Keywords: Addiction; Alcohol; Astrocyte; Excitatory amino acid transporter type 2; Glia; Glutamate; Striatum.
Figures
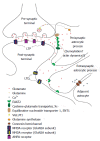
Figure 1
Fine regulation of glutamate levels and excitatory amino acid transporter 2 localization in neuron-glial interaction. The perisynaptic astrocytic process contains a higher concentration of EAAT2 immediately adjacent to the synapse. The EAP also contains EAAT2, but in lower concentrations. Glutamate spillover results in activation of GluN2B-containing NMDA receptors (increasing LTD response in the post-synaptic neuron), increased EAAT2 surface expression on and migration of EAPs, and activation of adjacent astrocytes via calcium waves propagated through connexin channels. LTP: Long term potentiation; LTD: Long term depression; EAAT2: Excitatory amino acid transporter 2; Xc-: Cysteine-glutamate transporter; ENT1: Equilibrative nucleoside transporter 1; VGLUT1: Vesicular glutamate transporter 1; NMDA: N-Nitrosodimethylamine; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; EAP: Extrasynaptic astrocytic process.
Similar articles
- Disentangling the Role of Astrocytes in Alcohol Use Disorder.
Adermark L, Bowers MS. Adermark L, et al. Alcohol Clin Exp Res. 2016 Sep;40(9):1802-16. doi: 10.1111/acer.13168. Epub 2016 Aug 1. Alcohol Clin Exp Res. 2016. PMID: 27476876 Free PMC article. Review. - Epigenetic Dysregulation in Alcohol-Associated Behaviors: Preclinical and Clinical Evidence.
Domi E, Barchiesi R, Barbier E. Domi E, et al. Curr Top Behav Neurosci. 2023 Jan 31. doi: 10.1007/7854_2022_410. Online ahead of print. Curr Top Behav Neurosci. 2023. PMID: 36717533 - Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder.
Goodwani S, Saternos H, Alasmari F, Sari Y. Goodwani S, et al. Neurosci Biobehav Rev. 2017 Jun;77:14-31. doi: 10.1016/j.neubiorev.2017.02.024. Epub 2017 Feb 24. Neurosci Biobehav Rev. 2017. PMID: 28242339 Free PMC article. Review. - Application of Novel Therapeutic Agents for CNS Injury: NAAG Peptidase Inhibitors.
Lyeth BG. Lyeth BG. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 38. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 38. PMID: 26269888 Free Books & Documents. Review. - Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury.
Fang Q, Hu WW, Wang XF, Yang Y, Lou GD, Jin MM, Yan HJ, Zeng WZ, Shen Y, Zhang SH, Xu TL, Chen Z. Fang Q, et al. Neuropharmacology. 2014 Feb;77:156-66. doi: 10.1016/j.neuropharm.2013.06.012. Epub 2013 Jun 18. Neuropharmacology. 2014. PMID: 23791559
Cited by
- Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders.
Nicosia N, Giovenzana M, Misztak P, Mingardi J, Musazzi L. Nicosia N, et al. Int J Mol Sci. 2024 Jun 13;25(12):6521. doi: 10.3390/ijms25126521. Int J Mol Sci. 2024. PMID: 38928227 Free PMC article. Review. - Alcohol and stress exposure across the lifespan are key risk factors for Alzheimer's Disease and cognitive decline.
Seemiller LR, Flores-Cuadra J, Griffith KR, Smith GC, Crowley NA. Seemiller LR, et al. Neurobiol Stress. 2024 Jan 4;29:100605. doi: 10.1016/j.ynstr.2024.100605. eCollection 2024 Mar. Neurobiol Stress. 2024. PMID: 38268931 Free PMC article. - Resident immune responses to spinal cord injury: role of astrocytes and microglia.
Brockie S, Zhou C, Fehlings MG. Brockie S, et al. Neural Regen Res. 2024 Aug 1;19(8):1678-1685. doi: 10.4103/1673-5374.389630. Epub 2023 Dec 11. Neural Regen Res. 2024. PMID: 38103231 Free PMC article. - Astrocyte Reactivity and Neurodegeneration in the Female Rat Brain Following Alcohol Dependence.
Guerin SP, Melbourne JK, Dang HQ, Shaji CA, Nixon K. Guerin SP, et al. Neuroscience. 2023 Oct 1;529:183-199. doi: 10.1016/j.neuroscience.2023.08.016. Epub 2023 Aug 19. Neuroscience. 2023. PMID: 37598836 Free PMC article. - Progress in Pathological and Therapeutic Research of HIV-Related Neuropathic Pain.
Hu Y, Liu J, Zhuang R, Zhang C, Lin F, Wang J, Peng S, Zhang W. Hu Y, et al. Cell Mol Neurobiol. 2023 Oct;43(7):3343-3373. doi: 10.1007/s10571-023-01389-7. Epub 2023 Jul 20. Cell Mol Neurobiol. 2023. PMID: 37470889 Review.
References
- Kandel ER, Schwarz MJ, Jessell TM. Principles of neural science. McGraw-Hill, 2000
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. - PubMed
- Barnett SC, Linington C. Myelination: do astrocytes play a role? Neuroscientist. 2013;19:442–450. - PubMed
- Pannasch U, Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 2013;36:405–417. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources