Positioning of centrioles is a conserved readout of Frizzled planar cell polarity signalling - PubMed (original) (raw)
Positioning of centrioles is a conserved readout of Frizzled planar cell polarity signalling
Jose Maria Carvajal-Gonzalez et al. Nat Commun. 2016.
Abstract
Planar cell polarity (PCP) signalling is a well-conserved developmental pathway regulating cellular orientation during development. An evolutionarily conserved pathway readout is not established and, moreover, it is thought that PCP mediated cellular responses are tissue-specific. A key PCP function in vertebrates is to regulate coordinated centriole/cilia positioning, a function that has not been associated with PCP in Drosophila. Here we report instructive input of Frizzled-PCP (Fz/PCP) signalling into polarized centriole positioning in Drosophila wings. We show that centrioles are polarized in pupal wing cells as a readout of PCP signalling, with both gain and loss-of-function Fz/PCP signalling affecting centriole polarization. Importantly, loss or gain of centrioles does not affect Fz/PCP establishment, implicating centriolar positioning as a conserved PCP-readout, likely downstream of PCP-regulated actin polymerization. Together with vertebrate data, these results suggest a unifying model of centriole/cilia positioning as a common downstream effect of PCP signalling from flies to mammals.
Figures
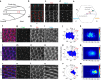
Figure 1. Centriole localization and positioning during PCP establishment in pupal wings.
(a) Illustration of pupal wing and its orientation (b–d) Sas4-labelled centrioles (cyan in b and monochrome d) are distributed in the junctional planes (X_–_Z plane—upper panels) (marked by Fmi staining, red in b, monochrome in c). Top panels are x_–_z sections of respective x_–_y views shown below. Scale bar, 10 mm. (e) Schematic representation of a pupal wing epithelial cell and the parameters used to study centriole positioning. (f–w) During pupal wing development, centriole localization changes. (f,l,r) Sas4 (green), Fmi stained in red, and actin (phalloidin) in blue, and the respective monochromes. (f–k) Before hair formation (29 h APF), centrioles are unpolarized in a central position in the apical portion of each cell (quantified in f,k). (l–q) At the onset of hair formation (31 h APF), centrioles begin to localize to the distal portion of each cell (quantified in p,q). (r–w) Subsequently, when hairs are fully present in all wing cells (32–34 h APF), centrioles appear to be polarized mostly to the distal sector of each cell (quantified in v,w). Scale bar, 10 mm. Red sectors in f,p,v—% within distal quadrant. Statistical analyses: rosette diagram distributions panel g versus p: _P<_0.0001; p versus v: _P<_0.0001 (χ2-test).
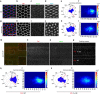
Figure 2. Centriole position is affected by PCP signalling.
(a–l) Fmi LOF, using _en_-driven fmi-IR knockdown affects centriole localization; (a,g) Dlg: red, cell outline; Sas4: green, centriole; actin (phalloidin): blue; and respective monochromes in a–d,h–j. Centrioles within cells in the en>fmi-IR area (g) are less polarized and are distributed more centrally, quantified in e–f and k–l, respectively. (m–t) Fz gain-of-function (GOF; see Methods), causes Fmi depolarization (see polarity vectors in p), and defects in centriole distribution (Fmi in red, monochrome in n; Sas4 in green, monochrome in o). Fz overexpressing cells have central distribution of centrioles (quantified in s–t; see also ROI: Fz-OE in p), as compared with WT areas of dpp>Fz-OE wings (quantified in h,i; see ROI: WT in p). Scale bar, 10 μm. Statistical analyses: centriole rosette diagrams, e versus k: _P<_0.0001; q versus s: _P<_0.0001 (χ2-test).
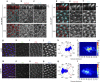
Figure 3. Centriole localization relative to trichome position.
(a–c) XZ and two XY optical sections of same wing area, showing polymerized actin (phalloidin, cyan) and acetylated tubulin staining (red, monochrome in b). At 29 h APF before actin-based hairs are formed, both actin and acetylated tubulin appear enriched at the apical portion of the pupal wing cells; two planes, apical (XY1) and subapical (XY2) are shown in the _XY_-axis (indicated in the XZ sections as black lines 1 and 2). (d–f) After hair formation (32 h APF), acetylated tubulin is enriched at the base of each hair and within the polymerized actin structure. XZ and two XY planes, apical (XY1) and subapical (XY2) are shown in the _XY_-axis from the same; stainings as in a. (g–l) Centriole localization in the mwh 1 mutant is less polarized than wt (in 32 h pupal wing cells; compared with Fig. 1v,w). Asl (green), Fmi (red) and phalloidin (blue) stainings and the respective monochromes are shown. k and l show centriole distribution quantifications in mwh 1 mutant, note shift to less polarized, central distribution most evident in heat map (l). (m–q) Overexpression of Sple (Sple-OE) causes reversal of cellular polarity and hair position, and accordingly also centriole positioning is inverted; quantifications in q,r. Asl staining position was used for quantifications in rosettes (k,q) and heat maps (l,r). Scale bar, 10 μm. Statistical analysis: centriole rosette diagrams, k versus Fig. 1w (wt control): _P<_0.001; q versus wt control: _P<_0.0001 (χ2-test).
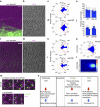
Figure 4. Loss or gain of centrioles does not affect core PCP factor localization.
(a–e) Imaging and quantifications (sed Methods) of Fmi staining in Sas4 RNAi-mediated knockdown (in cells marked with GFP) in the posterior compartment in 32–34 h APF pupal wings. en>Sas4 IR RNAi-mediated KD (a–b) did not disrupt Fmi localization as compared with the WT anterior compartment from same wings. (c,d) Quantifications of Fmi polarization using polarity vector angle orientation revealed no significant differences between en>Sas4 IR (_n_=1,872 cells from five independent wings) and WT cells (_n_=1,513 cells, five independent wings). NS: non-significant (_P=_0.655) (χ2-test)). (e) Polarity vector lengths (relative to Fmi fluorescence polarization) did not show significant differences between in Sas4 IR cells and adjacent WT cells (five independent wings). NS: non-significant. (f–j) Gain of centrioles (>2 per cell) through Asl overexpression (_n_=1,099 cells, three independent wings) in the posterior compartment (en>Asl), did not affect Fmi localization as compared with wild type from the same wings (_n_=1,096 cells, three independent wings). (h,i) Quantification of angle distribution of polarity vectors, and (j) polarity vector length. Note no significant changes between wt and en>Asl cells (_P=_0.783) (χ2-test). (k–m) Centriole positioning follows PCP core factor localization even when higher numbers of Asl-positive centrioles are present per cell. Quantifications are depicted in rosette (k) and heatmap (l) diagrams. (m,n) Specific examples of centriole localization in multi-centriolar cells, note several examples with 3–4 centrioles per cell (m, top), and two Asl-positive centrioles per cell (m, bottom); in WT only 1 centriole is stained by Asl for comparison (Asl in green; cf. to Fig. 1a,b). In all cases, centrioles remained close to the distal side of each cell (marked with Fmi; magenta). (n) Schematic representation of signalling pathways involved in centriole polarization related to planar cell polarity in vertebrates versus Drosophila. Scale bar, 10 μm.
Similar articles
- Centriole planar polarity assessment in Drosophila wings.
Garrido-Jimenez S, Roman AC, Alvarez-Barrientos A, Carvajal-Gonzalez JM. Garrido-Jimenez S, et al. Development. 2018 Dec 3;145(23):dev169326. doi: 10.1242/dev.169326. Development. 2018. PMID: 30389850 - Centriole positioning in epithelial cells and its intimate relationship with planar cell polarity.
Carvajal-Gonzalez JM, Mulero-Navarro S, Mlodzik M. Carvajal-Gonzalez JM, et al. Bioessays. 2016 Dec;38(12):1234-1245. doi: 10.1002/bies.201600154. Epub 2016 Oct 24. Bioessays. 2016. PMID: 27774671 Free PMC article. Review. - Two frizzled planar cell polarity signals in the Drosophila wing are differentially organized by the Fat/Dachsous pathway.
Hogan J, Valentine M, Cox C, Doyle K, Collier S. Hogan J, et al. PLoS Genet. 2011 Feb;7(2):e1001305. doi: 10.1371/journal.pgen.1001305. Epub 2011 Feb 17. PLoS Genet. 2011. PMID: 21379328 Free PMC article. - Diminished Expression of Fat and Dachsous PCP Proteins Impaired Centriole Planar Polarization in Drosophila.
Garrido-Jimenez S, Roman AC, Carvajal-Gonzalez JM. Garrido-Jimenez S, et al. Front Genet. 2019 Apr 12;10:328. doi: 10.3389/fgene.2019.00328. eCollection 2019. Front Genet. 2019. PMID: 31031805 Free PMC article. - Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt).
Yang Y, Mlodzik M. Yang Y, et al. Annu Rev Cell Dev Biol. 2015;31:623-46. doi: 10.1146/annurev-cellbio-100814-125315. Annu Rev Cell Dev Biol. 2015. PMID: 26566118 Free PMC article. Review.
Cited by
- Leucine repeat adaptor protein 1 interacts with Dishevelled to regulate gastrulation cell movements in zebrafish.
Cheng XN, Shao M, Li JT, Wang YF, Qi J, Xu ZG, Shi DL. Cheng XN, et al. Nat Commun. 2017 Nov 7;8(1):1353. doi: 10.1038/s41467-017-01552-x. Nat Commun. 2017. PMID: 29116181 Free PMC article. - Conserved and Divergent Principles of Planar Polarity Revealed by Hair Cell Development and Function.
Deans MR. Deans MR. Front Neurosci. 2021 Oct 18;15:742391. doi: 10.3389/fnins.2021.742391. eCollection 2021. Front Neurosci. 2021. PMID: 34733133 Free PMC article. Review. - Planar cell polarity induces local microtubule bundling for coordinated ciliary beating.
Nakayama S, Yano T, Namba T, Konishi S, Takagishi M, Herawati E, Nishida T, Imoto Y, Ishihara S, Takahashi M, Furuta K, Oiwa K, Tamura A, Tsukita S. Nakayama S, et al. J Cell Biol. 2021 Jul 5;220(7):e202010034. doi: 10.1083/jcb.202010034. J Cell Biol. 2021. PMID: 33929515 Free PMC article. - Junctional Adhesion Molecule 3 Expression in the Mouse Airway Epithelium Is Linked to Multiciliated Cells.
Mateos-Quiros CM, Garrido-Jimenez S, Álvarez-Hernán G, Diaz-Chamorro S, Barrera-Lopez JF, Francisco-Morcillo J, Roman AC, Centeno F, Carvajal-Gonzalez JM. Mateos-Quiros CM, et al. Front Cell Dev Biol. 2021 Jul 28;9:622515. doi: 10.3389/fcell.2021.622515. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34395412 Free PMC article. - Wnt/PCP Instructions for Cilia in Left-Right Asymmetry.
Wu J, Mlodzik M. Wu J, et al. Dev Cell. 2017 Mar 13;40(5):423-424. doi: 10.1016/j.devcel.2017.02.023. Dev Cell. 2017. PMID: 28292419 Free PMC article.
References
- Del Bigio M. R. Ependymal cells: biology and pathology. Acta Neuropathol. 119, 55–73 (2010). - PubMed
- Tissir F. & Goffinet A. M. Shaping the nervous system: role of the core planar cell polarity genes. Nat. Rev. Neurosci. 14, 525–535 (2013). - PubMed
- Nonaka S. et al.. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998). - PubMed
- Okada Y., Takeda S., Tanaka Y., Izpisua Belmonte J. C. & Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell 121, 633–644 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases