The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis - PubMed (original) (raw)
Review
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis
Daniel A Lim et al. Cold Spring Harb Perspect Biol. 2016.
Abstract
A large population of neural stem/precursor cells (NSCs) persists in the ventricular-subventricular zone (V-SVZ) located in the walls of the lateral brain ventricles. V-SVZ NSCs produce large numbers of neuroblasts that migrate a long distance into the olfactory bulb (OB) where they differentiate into local circuit interneurons. Here, we review a broad range of discoveries that have emerged from studies of postnatal V-SVZ neurogenesis: the identification of NSCs as a subpopulation of astroglial cells, the neurogenic lineage, new mechanisms of neuronal migration, and molecular regulators of precursor cell proliferation and migration. It has also become evident that V-SVZ NSCs are regionally heterogeneous, with NSCs located in different regions of the ventricle wall generating distinct OB interneuron subtypes. Insights into the developmental origins and molecular mechanisms that underlie the regional specification of V-SVZ NSCs have also begun to emerge. Other recent studies have revealed new cell-intrinsic molecular mechanisms that enable lifelong neurogenesis in the V-SVZ. Finally, we discuss intriguing differences between the rodent V-SVZ and the corresponding human brain region. The rapidly expanding cellular and molecular knowledge of V-SVZ NSC biology provides key insights into postnatal neural development, the origin of brain tumors, and may inform the development regenerative therapies from cultured and endogenous human neural precursors.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
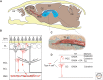
Figure 1.
Overview of adult mouse olfactory bulb (OB) neurogenesis from the ventricular–subventricular zone (V-SVZ). (A) Sagittal section through mouse head (calvarium is yellow). Neuroblasts (type A cells) born in the V-SVZ of the lateral ventricle (blue) migrate through a network of paths (red) into the rostral migratory stream (RMS), which enters the OB. Cells then leave the RMS (arrows, dashed lines) and migrate radially into the OB. Boxed area is shown enlarged in B. (B) Neuronal layers of OB. Migratory cells depart the RMS and differentiate into granule cells (GC) or periglomerular cells (PGC), which reside in the granule cell layer (GCL) and glomerular layer (GL), respectively (type A cells and differentiated interneurons are red). ORNs (small gray cells) in the olfactory epithelium (OE) project to the GL. The main projection neurons of the OB (mitral cells and tufted cells) are in gray. (C) Artists’ rendition of a chain of migratory type A cells. These chains are ensheathed by glial cells (type B cells, blue) and are associated with clusters of transit-amplifying cells (type C cells, green). (D) Diversity of OB interneurons. Type A cells differentiate into either PGCs or GCs, which can be distinguished by morphology, neurotransmitter (NT) phenotype, and markers. CC, Corpus callosum; Cx, cortex; CB, cerebellum; ORN, olfactory receptor neuron; MCL, mitral cell layer; ep, ependymal cell; TH, tyrosine hydroxylase.
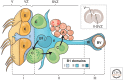
Figure 2.
Cellular composition of the ventricular–subventricular zone (V-SVZ). Coronal section of adult mouse brain is shown in the upper right. The V-SVZ region indicated by the black arrow is shown enlarged in the lower left. Type B1 cells (blue) are the astrocytes that serve as the V-SVZ stem cell. These can divide and produce type C cells (green), which are rapidly dividing, transit amplifying cells. Type C cells give rise to type A cells (red), the migratory neuroblasts. A blood vessel (BV, brown) is shown at the right. The apical surface of type B1 cells has a primary cilium and makes contact with the ventricle, which is at the left. These apical surfaces are found at the center of a “pinwheel” composed of multiciliated ependymal cells (yellow). The V-SVZ can be subdivided into three domains based on the structure and spatial arrangement of type B1 cells: Domain I (apical) contains the type B1 cells apical process and the body of ependymal cells; domain II (intermediate) contains the cell body of most type B1 cells, which are in contact with the type C and A cells; and domain III (basal) contains the B1 cell’s basal process with end-feet on blood vessels (see text for details).
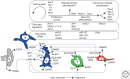
Figure 3.
Interactions of selected ventricular–subventricular zone (V-SVZ) niche factors and cell-intrinsic regulators. Solid arrows indicate the cellular source of a secreted factor, when known. Dotted lines indicate molecular interactions, both known and hypothetical. Blue dotted lines show interactions that may promote SVZ NSC self-renewal. Select transcription factors that may be involved in self-renewal, proliferation control, and specification of different cell types are in italics in the top inset above. Select chromatin-regulators are in the inset second from the top. Select noncoding RNAs are in the third inset from the top (see text for details).
Similar articles
- Embryonic Nkx2.1-expressing neural precursor cells contribute to the regional heterogeneity of adult V-SVZ neural stem cells.
Delgado RN, Lim DA. Delgado RN, et al. Dev Biol. 2015 Nov 15;407(2):265-74. doi: 10.1016/j.ydbio.2015.09.008. Epub 2015 Sep 24. Dev Biol. 2015. PMID: 26387477 Free PMC article. - Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenesis.
Cebrian-Silla A, Nascimento MA, Redmond SA, Mansky B, Wu D, Obernier K, Romero Rodriguez R, Gonzalez-Granero S, García-Verdugo JM, Lim DA, Álvarez-Buylla A. Cebrian-Silla A, et al. Elife. 2021 Jul 14;10:e67436. doi: 10.7554/eLife.67436. Elife. 2021. PMID: 34259628 Free PMC article. - Olig2 defines a subset of neural stem cells that produce specific olfactory bulb interneuron subtypes in the subventricular zone of adult mice.
Del Águila Á, Adam M, Ullom K, Shaw N, Qin S, Ehrman J, Nardini D, Salomone J, Gebelein B, Lu QR, Potter SS, Waclaw R, Campbell K, Nakafuku M. Del Águila Á, et al. Development. 2022 Mar 1;149(5):dev200028. doi: 10.1242/dev.200028. Epub 2022 Feb 28. Development. 2022. PMID: 35132995 Free PMC article. - Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain.
Obernier K, Alvarez-Buylla A. Obernier K, et al. Development. 2019 Feb 18;146(4):dev156059. doi: 10.1242/dev.156059. Development. 2019. PMID: 30777863 Free PMC article. Review. - Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis.
Fiorelli R, Azim K, Fischer B, Raineteau O. Fiorelli R, et al. Development. 2015 Jun 15;142(12):2109-20. doi: 10.1242/dev.119966. Development. 2015. PMID: 26081572 Review.
Cited by
- Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging.
Davinelli S, Medoro A, Ali S, Passarella D, Intrieri M, Scapagnini G. Davinelli S, et al. Curr Neuropharmacol. 2023;21(3):651-668. doi: 10.2174/1570159X21666221031103909. Curr Neuropharmacol. 2023. PMID: 36321225 Free PMC article. Review. - Vitamin C Deficiency Reduces Neurogenesis and Proliferation in the SVZ and Lateral Ventricle Extensions of the Young Guinea Pig Brain.
Jara N, Cifuentes M, Martínez F, González-Chavarría I, Salazar K, Ferrada L, Nualart F. Jara N, et al. Antioxidants (Basel). 2022 Oct 14;11(10):2030. doi: 10.3390/antiox11102030. Antioxidants (Basel). 2022. PMID: 36290753 Free PMC article. - Tsc2 coordinates neuroprogenitor differentiation.
Riley VA, Shankar V, Holmberg JC, Sokolov AM, Neckles VN, Williams K, Lyman R, Mackay TFC, Feliciano DM. Riley VA, et al. iScience. 2023 Nov 14;26(12):108442. doi: 10.1016/j.isci.2023.108442. eCollection 2023 Dec 15. iScience. 2023. PMID: 38107199 Free PMC article. - Developmental thyroid disruption permanently affects the neuroglial output in the murine subventricular zone.
Vancamp P, Le Blay K, Butruille L, Sébillot A, Boelen A, Demeneix BA, Remaud S. Vancamp P, et al. Stem Cell Reports. 2022 Mar 8;17(3):459-474. doi: 10.1016/j.stemcr.2022.01.002. Epub 2022 Feb 3. Stem Cell Reports. 2022. PMID: 35120623 Free PMC article. - Multiple steps characterise ventricular layer attrition to form the ependymal cell lining of the adult mouse spinal cord central canal.
Cañizares MA, Albors AR, Singer G, Suttie N, Gorkic M, Felts P, Storey KG. Cañizares MA, et al. J Anat. 2020 Feb;236(2):334-350. doi: 10.1111/joa.13094. Epub 2019 Oct 31. J Anat. 2020. PMID: 31670387 Free PMC article.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. 2007. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 25: 2827–2836. - PubMed
- Ahn S, Joyner AL. 2005. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897. - PubMed
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. 2012. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10: 76–87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous