Daily magnesium fluxes regulate cellular timekeeping and energy balance - PubMed (original) (raw)
. 2016 Apr 21;532(7599):375-9.
doi: 10.1038/nature17407. Epub 2016 Apr 13.
Affiliations
- PMID: 27074515
- PMCID: PMC4886825
- DOI: 10.1038/nature17407
Daily magnesium fluxes regulate cellular timekeeping and energy balance
Kevin A Feeney et al. Nature. 2016.
Abstract
Circadian clocks are fundamental to the biology of most eukaryotes, coordinating behaviour and physiology to resonate with the environmental cycle of day and night through complex networks of clock-controlled genes. A fundamental knowledge gap exists, however, between circadian gene expression cycles and the biochemical mechanisms that ultimately facilitate circadian regulation of cell biology. Here we report circadian rhythms in the intracellular concentration of magnesium ions, [Mg(2+)]i, which act as a cell-autonomous timekeeping component to determine key clock properties both in a human cell line and in a unicellular alga that diverged from each other more than 1 billion years ago. Given the essential role of Mg(2+) as a cofactor for ATP, a functional consequence of [Mg(2+)]i oscillations is dynamic regulation of cellular energy expenditure over the daily cycle. Mechanistically, we find that these rhythms provide bilateral feedback linking rhythmic metabolism to clock-controlled gene expression. The global regulation of nucleotide triphosphate turnover by intracellular Mg(2+) availability has potential to impact upon many of the cell's more than 600 MgATP-dependent enzymes and every cellular system where MgNTP hydrolysis becomes rate limiting. Indeed, we find that circadian control of translation by mTOR is regulated through [Mg(2+)]i oscillations. It will now be important to identify which additional biological processes are subject to this form of regulation in tissues of multicellular organisms such as plants and humans, in the context of health and disease.
Figures
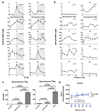
Extended Data Figure 1. Additional ICP-MS data and controls (Ostreococcus)
Inductively Coupled Plasma Mass Spectrometry analyses of cell lysates from 12 h:12 h light/dark cycles (a) or on the second day of constant light (b). P-values report significance by one-way ANOVA (mean±SEM, n=3). c, ICP-MS analyses on cell lysates compared with media control (no cells) and membrane fractions (lysed cells) (mean±SD plotted, n=2), indicating that magnesium signal in panel a and main Fig. 1 comes predominantly from the intracellular space. Groups are significantly different by one-way ANOVA (p<0.0001) Tukey's multiple comparisons p-values are indicated. d. Fluctuations in measured concentrations are not related to fluctuations in cell size over time. No significance of time as source of variation in cell size was observed by FACS analyses (mean±SD plotted, one-way ANOVA p-value is indicated, n=5).
Extended Data Figure 2. Additional ICP-MS data (U2OS cells) and controls
a. Inductively Coupled Plasma Mass Spectrometry analyses of U2OS cell extracts for several stable isotopes of several biologically relevant ions (mean±SEM, grey/black, n≥4), with insets showing standards that indicate linearity over the observed concentration ranges (mean ± %CV). We compared how well a straight-line + damped sine wave model (adapted from44) fit to each time series compared with a straight-line only (null hypothesis, no rhythm). The null hypothesis was preferred in each case except for Mg and K (analysed by Mg and K), where the sinusoidal fit with a circadian period was preferred (blue line, R2 and fit period±SEM are reported). b. Bmal1:luc bioluminescence data showing no effect of serum concentration on circadian rhythms in U2OS cells in the presence of B-27 supplement. c. Quantification of period and amplitude for data shown in b, mean±SEM (n=3), one-way ANOVA for period, p=0.79, one-way ANOVA for amplitude, p=0.01. d. Cellular impedance measurements indicate that U2OS cells do not proliferate upon reaching stationary phase under our assay conditions, reported doubling times (Td) were calculated from data collected between the dotted lines.
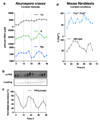
Extended Data Figure 3. Circadian rhythms of [Mg2+]i in Neurospora crassa and mouse fibroblasts
a. Circadian regulation of [Mg2+]i detected by ICP-MS in the fungus Neurospora crassa under constant darkness (mean±SEM, n=3). b. Representative (out of 3) FRQ immunoblot sampled in parallel. c. Quantification of FRQ abundance (mean±SEM, n=3). d. Circadian regulation of [Mg2+]i measured by luciferase-based assay is dependent upon CRYPTOCHROME in immortalised adult mouse fibroblasts under constant conditions (mean±SEM, n=3).
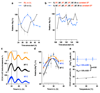
Extended Data Figure 4. Rhythms of [Mg2+]i entrain to relevant external cues and are temperature-compensated.
a. Inversion of 12 h : 12 h light/dark entrainment cycles is sufficient to entrain the phase of [Mg2+]i in Ostreococcus cells, measured by luciferase assay under constant light (mean±SEM, n=3). b. From the start of the experiment (S), 3 days of 12 h : 12 h temperature cycles between 32 and 37 ºC, followed a change to air medium (M) is sufficient to entrain the phase of [Mg2+]i in U2OS cells measured by ICP-MS over two circadian cycles under constant conditions (mean±SEM, n=3). c. Ostreococcus bioluminescence recordings (CCA1-LUC) at the indicated temperatures (n=8). Vertical dotted lines indicate sampling window for Mg2+ assays reported in panel d: assays performed during the second cycle under constant conditions show that circadian [Mg2+]i rhythms are temperature compensated (n=4). Each data set was fit with a Lorentzian curve to estimate peak [Mg2+]i. e. No significant difference in temperature compensation (Q10) between CCA1-LUC rhythms and the timing of the second Mg2+i peak; unpaired t-test p-value is reported.
Extended Data Figure 5. Human magnesium transporters and conservation in Ostreococcus tauri
a. Ubiquitously expressed human proteins with a clearly defined Mg2+ transport activity7 are listed. Note that many additional putative Mg2+-transporters are annotated, with several of these also being circadian-regulated in multiple mouse tissues. b. Expression profiles of Ostreococcus homologs of mammalian Mg2+ channels & transporters listed in (a), mined from publically available microarray data. aFrom BioGPS, bFrom CircaDB with JTK cycle p-value < 0.05. cFrom the Orcae service, d% sequence identity/similarity with human protein sequence (E-value). DELTA-BLAST performed using default settings. eFrom micro-array data shown in (b).
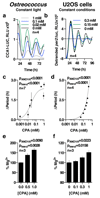
Extended Data Figure 6. Chronic CPA treatment dose-dependently lengthens period
Traces (a, b) of the CCA1-LUC (Ostreococcus) or per2:luc (U2OS cells) reporters, showing the effect of inhibition of magnesium transport by Co(NH3)5Cl2+ (CPA) upon period dose-response (c, d) and upon [Mg2+]i (e, f). All plots show mean±SEM, with replicate numbers (n) indicated, p-values report significance by 1-way ANOVA and post-test for linear trend.
Extended Data Figure 7. Period lengthening by CHA and SLC41 knockdown is dependent upon extracellular magnesium.
a. Extracellular magnesium-depletion and CHA act synergistically to lengthen circadian period in Ostreococcus cells (mean±SEM, n=4). b. Quantification of period lengthening by CHA at different concentrations of extracellular magnesium (mean±SEM, n=4), p-value for two-way ANOVA (interaction effect) is reported. c. Extracellular magnesium-depletion and CHA act synergistically to lengthen circadian period in human U2OS cells (mean±SEM, n=6). d. Quantification of period lengthening by CHA in Mg2+-depleted vs. normal media (mean±SEM, n=4), p-values for two-way ANOVA (interaction effect) and Fisher’s exact test are reported. e. Period lengthening due to knockdown of plasma membrane Mg2+/Na+ antiporter SLC41A1 is attenuated by depletion of extracellular magnesium (mean±SEM, n=8). f. Quantification of period lengthening due to knockdown of SLC41A1 in normal vs. Mg2+-depleted media (mean±SEM, n=8); two-way ANOVA interaction effect, p<0.0001, p-values for Sidak’s multiple comparisons test are also reported. g. Quantification of SLC41A1 knockdown efficacy, unpaired t-test p-values are reported, a representative immunoblot (of 3) is shown (mean±SEM, n=3).
Extended Data Figure 8. Bioluminescence data of wedge experiment
Peak expression phase of the clock protein CCA1 was analysed upon re-introduction of magnesium to cultures in low extracellular magnesium, to test whether the phase of cellular rhythms is dictated by the prior phase of entrainment or by this enforced transition from low to high [Mg2+]i. a, Bioluminescence traces showing that circadian rhythms in Ostreococcus are reversibly attenuated by depletion of extracellular Mg2+, and restored by Mg2+ wash-in. b,e. Bioluminescence traces from cells in low extracellular magnesium (b; 5 µM, e; 20 µM**)**with rhythms rescued by release into media containing normal physiological concentrations of magnesium at the indicated times (vertical dotted lines) in constant light (LL), compared to their respective controls where no magnesium was added in (blue traces). Data from 7-8 replicate wells are shown in each panel. c,f. Summary graphs where results from b,e are plotted in circadian wedge graphs: peak phases of CCA1-LUC rhythms in untreated control cells (grey dots) are compared with peak phase of rhythms reinstated by introduction of physiological magnesium following depletion to 5 µM (c, red dots) or 20 µM (f, orange dots), revealing that the phase of resulting rhythms is dictated solely by the phase of magnesium reintroduction (blue line). d,g. radial plots of phase shift (mean±SD, circumferential axis) depicted in panels c and f, versus phase prior to addition of Mg2+ to normal levels (old phase, radial axis and colour). The expected phase responses for type 0 resetting (black dotted line) and no resetting (red dotted line) are indicated. The goodness of fit (r2) and Y intercept (Y0) to the type 0 model are shown. Dose-dependent effects of intracellular magnesium on a critical clock parameter are confirmed by the observation that resetting is less strong when magnesium was reintroduced to cells adapted to intermediate levels of extracellular magnesium (e-g) compared to lowest extracellular magnesium (b-d).
Extended Data Figure 9. The effects of magnesium depletion and role of mTOR.
a. Extracellular lactate was measured in U2OS cells after 24 hours in Mg2+-depleted compared with normal media. b,c. Combined action of extracellular magnesium depletion and mTOR inhibition using torin1 (b, n=3) or rapamycin (c, n=6) to lengthen circadian period in U2OS cells is less than additive (mean±SEM). d,e. Quantification of period lengthening due to torin1 (d, n=3) and rapamycin (e, n=6) in Mg2+-depleted compared with normal media (mean±SEM). Note the apparent ‘ceiling effect’ at high concentrations of both drugs, such that Mg2+-depletion elicits no additional lengthening of cellular circadian period. Two-way ANOVA interaction effect: p<0.0001 for both drugs vs. Mg2+, selected p-values for Sidak’s multiple comparisons test are also reported (n.s., p>0.33).
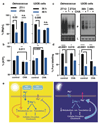
Extended Data Figure 10. Factors potentially contributing to maintenance of membrane electroneutrality in light of [Mg2+]i oscillations
a. Model indicating potential ion fluxes that might explain how clock-regulated [Mg2+]i oscillations impact on global cellular metabolism whilst membrane electroneutrality is maintained, during the day versus the night. The observed phase dependency of acute CHA was different between Ostreococcus and U2OS cells (Fig. 4a-c), and is consistent with the very different environmental niches inhabited by a marine alga compared with a peripheral human tissue. In Ostreococcus, CHA maintained [Mg2+]i at daytime levels when added prior to the normal trough, resulting in increased nighttime translation and a concomitant reduction in relative ATP levels. This result suggests that Ostreococcus pumps magnesium out of the cell during the dark period, against a large electrochemical potential gradient (magnesium is the second most abundant cation in seawater, at 50 mM in this study) in order to globally down-tune ATP turnover. In U2OS cells, CHA treatment significantly reduced [Mg2+]i accumulation and translation rates as well as significantly increasing ATP levels when added prior to the [Mg2+]i peak. Human cells inhabit an environment where nutrient availability is homeostatically regulated (0.8 mM magnesium in cell culture medium). As such, circadian regulation of increased magnesium transport into the cell during the feeding, active phase of day serves to facilitate higher metabolic rate constants. Please note that light has no direct effect on the clock in human peripheral cells, instead being mediated by systemic cues. b-c. [Mg2+]i oscillations persist in transcriptionally inactive Ostreococcus cells kept in constant darkness, as analysed by both ICP-MS (b) and luciferase assay (c), indicating that circadian regulation of ion transport can occur post-translationally in addition to its transcriptional regulation (mean±SEM, n=3 for ICP-MS data and n=4 for luciferase assays).
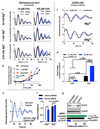
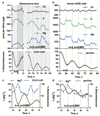
Figure 1. Conserved cellular rhythms in intracellular magnesium concentrations.
Time-series of lysates prepared from Ostreococcus (a, light/dark into constant light) or human U2OS cells (b, constant conditions) were subjected to Inductively Coupled Plasma Mass Spectrometry. Rhythms in magnesium concentration in cell lysates were confirmed with luciferase-based assays (c,d). Bioluminescence reporters for morning-phased clock gene expression were analysed in parallel (CCA1-LUC and Per2:luc) during both assays. All plots show mean±SEM, with replicate numbers (n) indicated. P-values report significance by 2-way ANOVA for time vs. interaction for each element (a,b) or 1-way ANOVA (c,d).
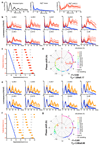
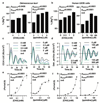
Figure 2. Chronic inhibition of magnesium transport leads to increased [Mg2+]i and long circadian period.
Chronic inhibition of magnesium transport by CHA or quinidine increases [Mg2+]i (a,b) and increases circadian period (c-f), traces and period dose-response of the CCA1-LUC (Ostreococcus) or Per2:luc (U2OS cells) reporters are shown. All plots are mean±SEM, with replicate numbers (n) indicated, p-values report significance by 1-way ANOVA and post-test for linear trend.
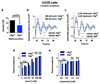
Figure 3. Reduced [Mg2+]i affects properties of cellular timekeeping and leads to an increase in ATP.
Bioluminescence traces showing reduced extracellular magnesium significantly affects amplitude and period length of circadian reporters in algal (a) and human cells (c). Low extracellular magnesium leads to decreased [Mg2+]i and increased [ATP]i in both cell types (b,d), measured after 4 days. All plots show mean±SEM, with replicate numbers (n) indicated, p-values (****p<0.0001, **p=0.01, *p=0.04) report significance by 1-way ANOVA (Ostreococcus) or t-test (U2OS).
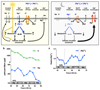
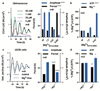
Figure 4. Magnesium transport phase-dependently affects cellular energy balance.
Cells were treated with CHA or vehicle during the second half of the day (black bars) or night (blue bars) to test acute effects on magnesium (a) and ATP (b) levels in both cell types. Incorporation of puromycin was analysed by western blot (c) then quantified to give relative translation rates at the indicated times (d). Mean±SEM are shown, n=3, t-test significance reported (n.s., p>0.18). e, Simplified model for a feedback mechanism between circadian [Mg2+]i rhythms and the clockwork to temporally regulate global cellular metabolism; orange arrow represents extracellular stimuli.
Comment in
- Yes, circadian rhythms actually do affect almost everything.
Dunlap JC, Loros JJ. Dunlap JC, et al. Cell Res. 2016 Jul;26(7):759-60. doi: 10.1038/cr.2016.65. Epub 2016 May 31. Cell Res. 2016. PMID: 27241553 Free PMC article.
Similar articles
- Intracellular magnesium and the rhythms of life.
van Ooijen G, O'Neill JS. van Ooijen G, et al. Cell Cycle. 2016 Nov 16;15(22):2997-2998. doi: 10.1080/15384101.2016.1214030. Epub 2016 Jul 27. Cell Cycle. 2016. PMID: 27463066 Free PMC article. No abstract available. - Interplay between cellular redox oscillations and circadian clocks.
Rey G, Reddy AB. Rey G, et al. Diabetes Obes Metab. 2015 Sep;17 Suppl 1:55-64. doi: 10.1111/dom.12519. Diabetes Obes Metab. 2015. PMID: 26332969 Review. - Rewiring of liver diurnal transcriptome rhythms by triiodothyronine (T3) supplementation.
de Assis LVM, Harder L, Lacerda JT, Parsons R, Kaehler M, Cascorbi I, Nagel I, Rawashdeh O, Mittag J, Oster H. de Assis LVM, et al. Elife. 2022 Jul 27;11:e79405. doi: 10.7554/eLife.79405. Elife. 2022. PMID: 35894384 Free PMC article. - The circadian clock coordinates ribosome biogenesis.
Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, Gachon F. Jouffe C, et al. PLoS Biol. 2013;11(1):e1001455. doi: 10.1371/journal.pbio.1001455. Epub 2013 Jan 3. PLoS Biol. 2013. PMID: 23300384 Free PMC article. - The role of spatiotemporal organization and dynamics of clock complexes in circadian regulation.
Yuan Y, Xiao Y, Yadlapalli S. Yuan Y, et al. Curr Opin Cell Biol. 2022 Oct;78:102129. doi: 10.1016/j.ceb.2022.102129. Epub 2022 Sep 18. Curr Opin Cell Biol. 2022. PMID: 36126370 Free PMC article. Review.
Cited by
- Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005-2016.
Ikonte CJ, Mun JG, Reider CA, Grant RW, Mitmesser SH. Ikonte CJ, et al. Nutrients. 2019 Oct 1;11(10):2335. doi: 10.3390/nu11102335. Nutrients. 2019. PMID: 31581561 Free PMC article. - NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1.
Shen Y, Endale M, Wang W, Morris AR, Francey LJ, Harold RL, Hammers DW, Huo Z, Partch CL, Hogenesch JB, Wu ZH, Liu AC. Shen Y, et al. PLoS Genet. 2021 Nov 22;17(11):e1009933. doi: 10.1371/journal.pgen.1009933. eCollection 2021 Nov. PLoS Genet. 2021. PMID: 34807912 Free PMC article. - CikA, an Input Pathway Component, Senses the Oxidized Quinone Signal to Generate Phase Delays in the Cyanobacterial Circadian Clock.
Kim P, Porr B, Mori T, Kim YS, Johnson CH, Diekman CO, Kim YI. Kim P, et al. J Biol Rhythms. 2020 Jun;35(3):227-234. doi: 10.1177/0748730419900868. Epub 2020 Jan 27. J Biol Rhythms. 2020. PMID: 31983264 Free PMC article. - In-depth Characterization of Firefly Luciferase as a Reporter of Circadian Gene Expression in Mammalian Cells.
Feeney KA, Putker M, Brancaccio M, O'Neill JS. Feeney KA, et al. J Biol Rhythms. 2016 Dec;31(6):540-550. doi: 10.1177/0748730416668898. Epub 2016 Oct 10. J Biol Rhythms. 2016. PMID: 28112045 Free PMC article. - Circadian physiology of metabolism.
Panda S. Panda S. Science. 2016 Nov 25;354(6315):1008-1015. doi: 10.1126/science.aah4967. Science. 2016. PMID: 27885007 Free PMC article. Review.
References
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 093734/WT_/Wellcome Trust/United Kingdom
- MC_UP_1201/4/MRC_/Medical Research Council/United Kingdom
- 093734/Z/10/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous