Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia - PubMed (original) (raw)
Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia
Vicente Hernandez-Rabaza et al. J Neuroinflammation. 2016.
Abstract
Background: Hyperammonemia induces neuroinflammation and increases GABAergic tone in the cerebellum which contributes to cognitive and motor impairment in hepatic encephalopathy (HE). The link between neuroinflammation and GABAergic tone remains unknown. New treatments reducing neuroinflammation and GABAergic tone could improve neurological impairment. The aims were, in hyperammonemic rats, to assess whether: (a) Enhancing endogenous anti-inflammatory mechanisms by sulforaphane treatment reduces neuroinflammation and restores learning and motor coordination. (b) Reduction of neuroinflammation by sulforaphane normalizes extracellular GABA and glutamate-NO-cGMP pathway and identify underlying mechanisms. (c) Identify steps by which hyperammonemia-induced microglial activation impairs cognitive and motor function and how sulforaphane restores them.
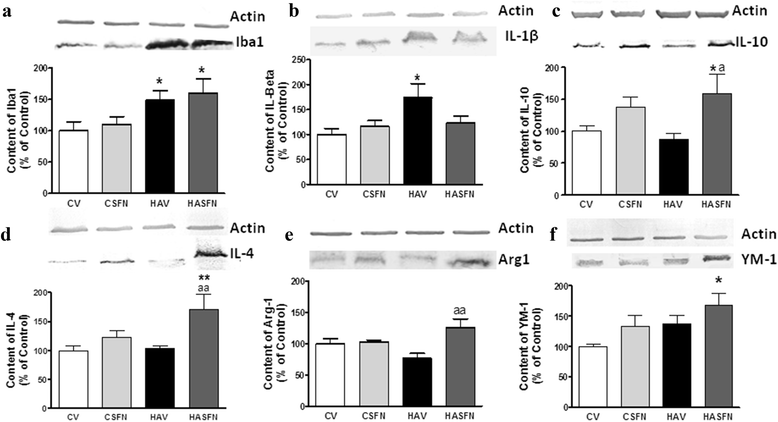
Methods: We analyzed in control and hyperammonemic rats, treated or not with sulforaphane, (a) learning in the Y maze; (b) motor coordination in the beam walking; (c) glutamate-NO-cGMP pathway and extracellular GABA by microdialysis; (d) microglial activation, by analyzing by immunohistochemistry or Western blot markers of pro-inflammatory (M1) (IL-1b, Iba-1) and anti-inflammatory (M2) microglia (Iba1, IL-4, IL-10, Arg1, YM-1); and (e) membrane expression of the GABA transporter GAT-3.
Results: Hyperammonemia induces activation of astrocytes and microglia in the cerebellum as assessed by immunohistochemistry. Hyperammonemia-induced neuroinflammation is associated with increased membrane expression of the GABA transporter GAT-3, mainly in activated astrocytes. This is also associated with increased extracellular GABA in the cerebellum and with motor in-coordination and impaired learning ability in the Y maze. Sulforaphane promotes polarization of microglia from the M1 to the M2 phenotype, reducing IL-1b and increasing IL-4, IL-10, Arg1, and YM-1 in the cerebellum. This is associated with astrocytes deactivation and normalization of GAT-3 membrane expression, extracellular GABA, glutamate-nitric oxide-cGMP pathway, and learning and motor coordination.
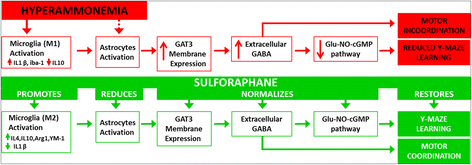
Conclusions: Neuroinflammation increases GABAergic tone in the cerebellum by increasing GAT-3 membrane expression. This impairs motor coordination and learning in the Y maze. Sulforaphane could be a new therapeutic approach to improve cognitive and motor function in hyperammonemia, hepatic encephalopathy, and other pathologies associated with neuroinflammation by promoting microglia differentiation from M1 to M2.
Keywords: GABA; GAT-3; Hepatic encephalopathy; Hyperammonemia; Learning; Microglia; Motor in-coordination; Neuroinflammation; Sulforaphane.
Figures
Fig. 1
Scheme showing the experimental design
Fig. 2
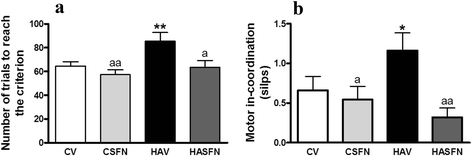
Treatment with sulforaphane restores learning ability in the Y maze and motor coordination in hyperammonemic rats. Control (C) and hyperammonemic (HA) rats treated with vehicle (V) or sulforaphane (SFN) were subjected to the conditional discrimination learning test in the Y maze (a) and the beam walking (b) tests. Values are the mean ± SEM of 11 rats per group in (a) and 22 rats per group in (b). Values significantly different from control rats are indicated by asterisks. Values significantly different from hyperammonemic rats are indicated by “_a_”. *p < 0.05; **p < 0.01; “_a_” p < 005; “_aa_” p < 0.01
Fig. 3
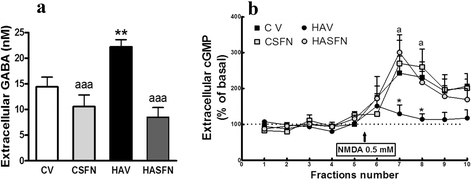
Sulforaphane normalizes extracellular GABA and the function of the glutamate-NO-cGMP pathway in the cerebellum of hyperammonemic rats. Control (C) and hyperammonemic (HA) rats treated with vehicle (V) or (SFN) were subjected to in vivo microdialysis in the cerebellum. a Extracellular GABA was measured by HPLC in the five initial samples of each rat. b After taking five samples to determine basal levels of cGMP, NMDA 0.5 mM was administered in the perfusion stream, and cGMP was determined in the following five samples to determine to function of the glutamate-nitric oxide-cGMP. Values are the mean ± SEM of six to eight rats per group. Values significantly different from control rats are indicated by asterisks. Values significantly different from hyperammonemic rats are indicated by “_a_”. **p < 0.01; “_a_” p < 0.05; “_aaa_” p < 0.001
Fig. 4
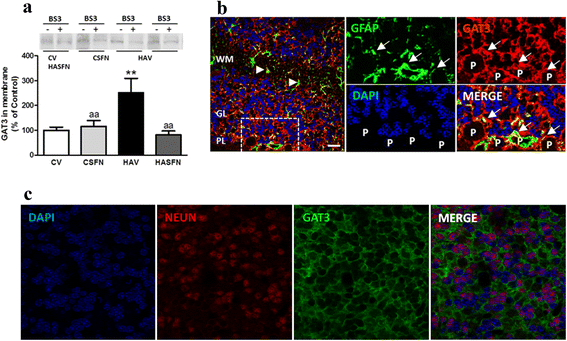
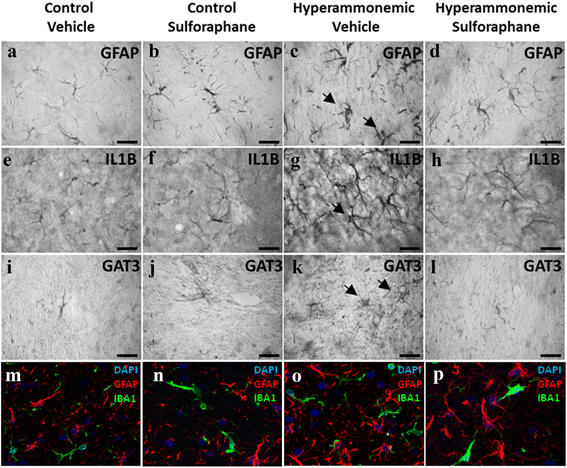
Hyperammonemia increases and treatment with sulforaphane normalizes membrane expression of GAT-3 in the cerebellum. a Membrane expression of GAT-3 was analyzed by Western blot after cross-linking with BS3. A typical image of the blots with and without BS3 is shown. Membrane expression was quantified as described in methods. Values are the mean ± SEM of six rats per group. Values significantly different from control rats are indicated by asterisks. Values significantly different from hyperammonemic rats are indicated by “_a_”. **p < 0.01; “_aa_” p < 0.01. b Double immunofluorescence staining of GFAP (green) and GAT-3 (red). Nuclei are stained with DAPI (blue). In the merged image, co-localization of GAT-3 and GFAP appears in yellow. It can be seen that GAT-3 is expressed in astrocytes surrounding Purkinje neurons (P). c Double immunofluorescence staining of Neun (red) and GAT-3 (green). Nuclei are stained with DAPI (blue). In the merged image, no co-localization of GAT-3 and Neun is observed
Fig. 5
Hyperammonemia increases markers of M1 (pro-inflammatory) form of microglia and treatment with sulforaphane increases markers of M2 (anti-inflammatory) form of microglia in hyperammonemic rats. Cerebellar homogenates from control (C) and hyperammonemic (HA) rats treated with vehicle (V) or sulforaphane (SFN) were subjected to electrophoresis and Western blot using antibodies against a Iba1, marker of microglial activation; b the pro-inflammatory cytokine IL-1b, marker of M1 microglia; and against the markers of M2 microglia IL-10 (c), IL-4 (d), YM-1 (e), and Arginase 1 (f). Representative images of the blots are shown for each protein. Values are the mean ± SEM of six to nine rats per group. Values significantly different from control rats are indicated by asterisks. Values significantly different from hyperammonemic rats are indicated by “_a_”. *p < 0.05; **p < 0.01; “_a_” p < 0.05; “_aa_” p < 0.01
Fig. 6
Hyperammonemia induces and treatment with sulforaphane reduces activation of astrocytes, which express IL-1b and GAT-3. Immunohistochemistry was performed as indicated in methods with DAB staining using antibodies against GFAP (a–d), IL-1b (e–h), or GAT-3 (i–l). Hyperammonemic rats show an altered morphology of astrocytes stained with GFAP (indicated by arrows), indicating activation (c). Treatment with SFN reduces astrocyte activation and normalizes the morphology (d). Activated astrocytes in hyperammonemic rats show increased labeling of IL-1b (g) and GAT-3 (k), which are normalized by treatment with SFN (h, l). m–p Double immunofluorescence staining of GFAP (red) and Iba-1 (green). Nuclei are stained with DAPI (blue). In the merged image, no co-localization of GFAP and Iba-1 is observed
Fig. 7
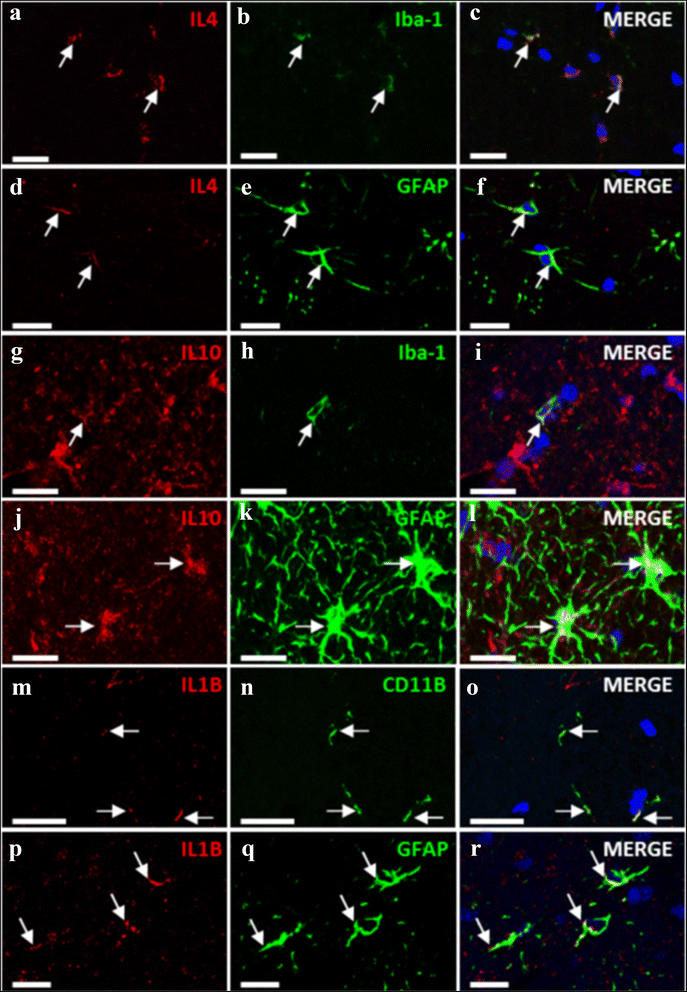
IL-4, IL-10, and IL-1b are present both in microglia and astrocytes. The images show immunostaining by double fluorescence of astrocytes (GFAP) or microglia (Iba-1 or CD11b) and IL-4 (a–f); IL-10 (g–l), or IL-1b (m–r). Arrows indicate co-localization of the labeling of the IL with the corresponding cell type marker
Fig. 8
Proposed sequences of events by which hyperammonemia-induced neuroinflammation leads to cognitive and motor impairment (in red) and treatment with sulforaphane restore them (in green). See details in “Discussion” section
Similar articles
- Infliximab reduces peripheral inflammation, neuroinflammation, and extracellular GABA in the cerebellum and improves learning and motor coordination in rats with hepatic encephalopathy.
Dadsetan S, Balzano T, Forteza J, Agusti A, Cabrera-Pastor A, Taoro-Gonzalez L, Hernandez-Rabaza V, Gomez-Gimenez B, ElMlili N, Llansola M, Felipo V. Dadsetan S, et al. J Neuroinflammation. 2016 Sep 13;13(1):245. doi: 10.1186/s12974-016-0710-8. J Neuroinflammation. 2016. PMID: 27623772 Free PMC article. - Increasing extracellular cGMP in cerebellum in vivo reduces neuroinflammation, GABAergic tone and motor in-coordination in hyperammonemic rats.
Cabrera-Pastor A, Balzano T, Hernández-Rabaza V, Malaguarnera M, Llansola M, Felipo V. Cabrera-Pastor A, et al. Brain Behav Immun. 2018 Mar;69:386-398. doi: 10.1016/j.bbi.2017.12.013. Epub 2017 Dec 27. Brain Behav Immun. 2018. PMID: 29288802 - Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane.
Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M, Felipo V. Hernández-Rabaza V, et al. J Neuroinflammation. 2016 Feb 16;13:41. doi: 10.1186/s12974-016-0505-y. J Neuroinflammation. 2016. PMID: 26883214 Free PMC article. - Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications.
Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, García-García R, Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM, Leone P, Felipo V. Cabrera-Pastor A, et al. Acta Physiol (Oxf). 2019 Jun;226(2):e13270. doi: 10.1111/apha.13270. Epub 2019 Mar 22. Acta Physiol (Oxf). 2019. PMID: 30830722 Review. - Neuroinflammation alters GABAergic neurotransmission in hyperammonemia and hepatic encephalopathy, leading to motor incoordination. Mechanisms and therapeutic implications.
Llansola M, Arenas YM, Sancho-Alonso M, Mincheva G, Palomares-Rodriguez A, Doverskog M, Izquierdo-Altarejos P, Felipo V. Llansola M, et al. Front Pharmacol. 2024 Mar 15;15:1358323. doi: 10.3389/fphar.2024.1358323. eCollection 2024. Front Pharmacol. 2024. PMID: 38560359 Free PMC article. Review.
Cited by
- Infliximab reduces peripheral inflammation, neuroinflammation, and extracellular GABA in the cerebellum and improves learning and motor coordination in rats with hepatic encephalopathy.
Dadsetan S, Balzano T, Forteza J, Agusti A, Cabrera-Pastor A, Taoro-Gonzalez L, Hernandez-Rabaza V, Gomez-Gimenez B, ElMlili N, Llansola M, Felipo V. Dadsetan S, et al. J Neuroinflammation. 2016 Sep 13;13(1):245. doi: 10.1186/s12974-016-0710-8. J Neuroinflammation. 2016. PMID: 27623772 Free PMC article. - Sulforaphane, an Nrf-2 Agonist, Modulates Oxidative Stress and Inflammation in a Rat Model of Cuprizone-Induced Cardiotoxicity and Hepatotoxicity.
Ibrahim Fouad G. Ibrahim Fouad G. Cardiovasc Toxicol. 2023 Jan;23(1):46-60. doi: 10.1007/s12012-022-09776-0. Epub 2023 Jan 17. Cardiovasc Toxicol. 2023. PMID: 36650404 Free PMC article. - Sulforaphane diminishes moonlighting of pyruvate kinase M2 and interleukin 1β expression in M1 (LPS) macrophages.
Bahiraii S, Brenner M, Yan F, Weckwerth W, Heiss EH. Bahiraii S, et al. Front Immunol. 2022 Aug 2;13:935692. doi: 10.3389/fimmu.2022.935692. eCollection 2022. Front Immunol. 2022. PMID: 35983049 Free PMC article. - Hepatic encephalopathy: Lessons from preclinical studies.
Lima LCD, Miranda AS, Ferreira RN, Rachid MA, Simões E Silva AC. Lima LCD, et al. World J Hepatol. 2019 Feb 27;11(2):173-185. doi: 10.4254/wjh.v11.i2.173. World J Hepatol. 2019. PMID: 30820267 Free PMC article. Review. - Increased levels and activation of the IL-17 receptor in microglia contribute to enhanced neuroinflammation in cerebellum of hyperammonemic rats.
Arenas YM, López-Gramaje A, Montoliu C, Llansola M, Felipo V. Arenas YM, et al. Biol Res. 2024 Apr 27;57(1):18. doi: 10.1186/s40659-024-00504-2. Biol Res. 2024. PMID: 38671534 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous