Seed Priming Alters the Production and Detoxification of Reactive Oxygen Intermediates in Rice Seedlings Grown under Sub-optimal Temperature and Nutrient Supply - PubMed (original) (raw)
Seed Priming Alters the Production and Detoxification of Reactive Oxygen Intermediates in Rice Seedlings Grown under Sub-optimal Temperature and Nutrient Supply
Saddam Hussain et al. Front Plant Sci. 2016.
Abstract
The production and detoxification of reactive oxygen intermediates (ROIs) play an important role in the plant response to nutrient and environmental stresses. The present study demonstrated the behavior of growth, ROIs-production and their detoxification in primed and non-primed rice seedlings under chilling stress (18°C) and nitrogen-(N), phosphorus-(P), or potassium-(K) deprivation. The results revealed that chilling stress as well as deprivation of any mineral nutrient severely hampered the seedling growth of rice, however, seed priming treatments (particularly selenium- or salicylic acid-priming), were effective in enhancing the rice growth under stress conditions. The N-deprivation caused the maximum reduction in shoot growth, while the root growth was only decreased by P- or K-deprivation. Although, N-deprivation enhanced the root length of rice, the root fresh weight was unaffected. Rate of lipid peroxidation as well as the production of ROIs, was generally increased under stress conditions; the K-deprived seedlings recorded significantly lower production of ROIs than N- or P-deprived seedlings. The responses of enzymatic and non-enzymatic antioxidants in rice seedlings to chilling stress were variable with nutrient management regime. All the seed priming were found to trigger or at least maintain the antioxidant defense system of rice seedlings. Notably, the levels of ROIs were significantly reduced by seed priming treatments, which were concomitant with the activities of ROIs-producing enzymes (monoamine oxidase and xanthine oxidase), under all studied conditions. Based on these findings, we put forward the hypothesis that along with role of ROIs-scavenging enzymes, the greater tolerance of primed rice seedlings can also be due to the reduced activity of ROIs-producing enzymes.
Keywords: antioxidant defense system; chilling stress; monoamine oxidase; nutrient deprivation; reactive oxygen intermediates; seed priming.
Figures
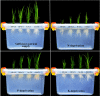
FIGURE 1
Pictorial view of primed and non-primed rice seedlings grown under chilling stress and different nutrient management regimes. Photographs were taken at 15 DAS for treatments under chilling stress (18°C). NP+CS: no priming and 18°C temperature, HP+CS: hydropriming and 18°C temperature, Se+CS: selenium priming and 18°C temperature, SA+CS: salicylic acid priming and 18°C temperature.
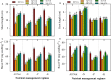
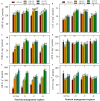
FIGURE 2
Shoot length (A), root length (B), shoot fresh weight (C), and root fresh weight (D) of primed and non-primed rice seedlings as influenced by chilling stress and different nutrient management regimes. Vertical bars above mean indicate standard error of six replicates. Small alphabetical letters (a, b, c…) above means show the differences (P ≤ 0.05) among treatments with in a nutrient management regime, while capital alphabetical letters (A, B, C…) reveal the differences (P ≤ 0.05) among different nutrient management regimes. NP+Cn: no priming and 28°C temperature, NP+CS: no priming and 18°C temperature, HP+CS: hydropriming and 18°C temperature, Se+CS: selenium priming and 18°C temperature, SA+CS: salicylic acid priming and 18°C temperature, All Nut: sufficient nutrient supply, -N: nitrogen-deprivation, -P: phosphorus-deprivation, -K: potassium-deprivation.
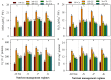
FIGURE 3
Lipid peroxidation and accumulation of reactive oxygen intermediates (ROIs) in primed and non-primed rice seedlings under chilling stress and different nutrient management regimes. MDA content (A), H2O2 (B), O2•- (C), and OH- (D). Vertical bars above mean indicate standard error of six replicates. Small alphabetical letters (a, b, c…) above means show the differences (P ≤ 0.05) among treatments with in a nutrient management regime, while capital alphabetical letters (A, B, C…) reveal the differences (P ≤ 0.05) among different nutrient management regimes. Description of treatments is given in Figure 2.
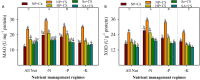
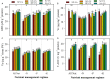
FIGURE 4
Activities of monoamine oxidase (MAO) (A) and XOD (B) in primed and non-primed rice seedlings under chilling stress and different nutrient management regimes. Vertical bars above mean indicate standard error of six replicates. Small alphabetical letters (a, b, c…) above means show the differences (P ≤ 0.05) among treatments with in a nutrient management regime, while capital alphabetical letters (A, B, C…) reveal the differences (P ≤ 0.05) among different nutrient management regimes. Description of treatments is given in Figure 2.
FIGURE 5
Activities of enzymatic antioxidants in primed and non-primed rice seedlings under chilling stress and different nutrient management regimes. SOD (A), CAT (B), POD (C), GPX (D), GR (E), and GST (F). Vertical bars above mean indicate standard error of six replicates. Small alphabetical letters (a, b, c…) above means show the differences (P ≤ 0.05) among treatments with in a nutrient management regime, while capital alphabetical letters (A, B, C…) reveal the differences (P ≤ 0.05) among different nutrient management regimes. Description of treatments is given in Figure 2.
FIGURE 6
Non-enzymatic antioxidants and total antioxidant capability of primed and non-primed rice seedlings as influenced by chilling stress and different nutrient management regimes. GSH (A), Vc (B), Ve (C), and T-AOC (D). Vertical bars above mean indicate standard error of six replicates. Small alphabetical letters (a, b, c…) above means show the differences (P ≤ 0.05) among treatments with in a nutrient management regime, while capital alphabetical letters (A, B, C…) reveal the differences (P ≤ 0.05) among different nutrient management regimes. Description of treatments is given in Figure 2.
Similar articles
- Seed Priming Improved Antioxidant Defense System and Alleviated Ni-Induced Adversities in Rice Seedlings Under N, P, or K Deprivation.
Khan F, Hussain S, Khan S, Geng M. Khan F, et al. Front Plant Sci. 2020 Sep 3;11:565647. doi: 10.3389/fpls.2020.565647. eCollection 2020. Front Plant Sci. 2020. PMID: 33013986 Free PMC article. - Coordinated effects of lead toxicity and nutrient deprivation on growth, oxidative status, and elemental composition of primed and non-primed rice seedlings.
Khan F, Hussain S, Tanveer M, Khan S, Hussain HA, Iqbal B, Geng M. Khan F, et al. Environ Sci Pollut Res Int. 2018 Jul;25(21):21185-21194. doi: 10.1007/s11356-018-2262-1. Epub 2018 May 17. Environ Sci Pollut Res Int. 2018. PMID: 29774513 - Physiological and Biochemical Mechanisms of Seed Priming-Induced Chilling Tolerance in Rice Cultivars.
Hussain S, Khan F, Hussain HA, Nie L. Hussain S, et al. Front Plant Sci. 2016 Feb 9;7:116. doi: 10.3389/fpls.2016.00116. eCollection 2016. Front Plant Sci. 2016. PMID: 26904078 Free PMC article. - Effects of various seed priming on morphological, physiological, and biochemical traits of rice under chilling stress.
Zhang H, Zhang X, Gao G, Ali I, Wu X, Tang M, Chen L, Jiang L, Liang T. Zhang H, et al. Front Plant Sci. 2023 Mar 13;14:1146285. doi: 10.3389/fpls.2023.1146285. eCollection 2023. Front Plant Sci. 2023. PMID: 36993861 Free PMC article. - Pre-sowing Seed Treatments in Direct-seeded Early Rice: Consequences for Emergence, Seedling Growth and Associated Metabolic Events under Chilling Stress.
Wang W, Chen Q, Hussain S, Mei J, Dong H, Peng S, Huang J, Cui K, Nie L. Wang W, et al. Sci Rep. 2016 Jan 19;6:19637. doi: 10.1038/srep19637. Sci Rep. 2016. PMID: 26782108 Free PMC article.
Cited by
- Seed Priming Improved Antioxidant Defense System and Alleviated Ni-Induced Adversities in Rice Seedlings Under N, P, or K Deprivation.
Khan F, Hussain S, Khan S, Geng M. Khan F, et al. Front Plant Sci. 2020 Sep 3;11:565647. doi: 10.3389/fpls.2020.565647. eCollection 2020. Front Plant Sci. 2020. PMID: 33013986 Free PMC article. - Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review.
D'Amato R, Regni L, Falcinelli B, Mattioli S, Benincasa P, Dal Bosco A, Pacheco P, Proietti P, Troni E, Santi C, Businelli D. D'Amato R, et al. J Agric Food Chem. 2020 Apr 8;68(14):4075-4097. doi: 10.1021/acs.jafc.0c00172. Epub 2020 Mar 25. J Agric Food Chem. 2020. PMID: 32181658 Free PMC article. Review. - Maize Tolerance against Drought and Chilling Stresses Varied with Root Morphology and Antioxidative Defense System.
Hussain HA, Men S, Hussain S, Zhang Q, Ashraf U, Anjum SA, Ali I, Wang L. Hussain HA, et al. Plants (Basel). 2020 Jun 6;9(6):720. doi: 10.3390/plants9060720. Plants (Basel). 2020. PMID: 32517168 Free PMC article. - Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress.
Shemi R, Wang R, Gheith EMS, Hussain HA, Hussain S, Irfan M, Cholidah L, Zhang K, Zhang S, Wang L. Shemi R, et al. Sci Rep. 2021 Feb 4;11(1):3195. doi: 10.1038/s41598-021-82264-7. Sci Rep. 2021. PMID: 33542287 Free PMC article. - Selenium-Ethylene Interplay in Postharvest Life of Cut Flowers.
Costa LC, Luz LM, Nascimento VL, Araujo FF, Santos MNS, França CFM, Silva TP, Fugate KK, Finger FL. Costa LC, et al. Front Plant Sci. 2020 Dec 17;11:584698. doi: 10.3389/fpls.2020.584698. eCollection 2020. Front Plant Sci. 2020. PMID: 33391299 Free PMC article. Review.
References
- Bailly C., Benamar A., Corbineau F., Côme D. (2000). Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci. Res. 10 35–42. 10.1016/j.plantsci.2011.06.003 - DOI
- Bailly C., Benamar A., Corbineau F., Dome D. (1996). Changes in malondialdehyde contents and in superoxide dismutase, catalase, glutathione reductase activities in sunflower seeds related to accelerated seed aging. Physiol. Plant 97 104–110. 10.1111/j.1399-3054.1996.tb00485.x - DOI
- Bhattachrjee S. (2005). Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plant. Curr. Sci. 89 1113–1121.
LinkOut - more resources
Full Text Sources
Other Literature Sources