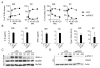An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells - PubMed (original) (raw)
An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells
Ivan Zanoni et al. Science. 2016.
Abstract
Dendritic cells (DCs) use pattern recognition receptors to detect microorganisms and activate protective immunity. These cells and receptors are thought to operate in an all-or-nothing manner, existing in an immunologically active or inactive state. Here, we report that encounters with microbial products and self-encoded oxidized phospholipids (oxPAPC) induce an enhanced DC activation state, which we call "hyperactive." Hyperactive DCs induce potent adaptive immune responses and are elicited by caspase-11, an enzyme that binds oxPAPC and bacterial lipopolysaccharide (LPS). oxPAPC and LPS bind caspase-11 via distinct domains and elicit different inflammasome-dependent activities. Both lipids induce caspase-11-dependent interleukin-1 release, but only LPS induces pyroptosis. The cells and receptors of the innate immune system can therefore achieve different activation states, which may permit context-dependent responses to infection.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Fig. 1. oxPAPC does not induce TLR4 signaling
(A) MΦs or DCs were treated with LPS or oxPAPC for the indicated time points. TLR4 dimerization and endocytosis was measured by flow cytometry. Line graph represents means and standard deviations of four replicates. (B) MΦs or DCs were treated with LPS or oxPAPC. Cytokine production was analyzed 18 hours later. Means and standard deviations of four replicates are shown. (C) Myddosome formation in iMΦs was assessed at the indicated time points after treatment with LPS or oxPAPC by co-immunoprecipitation (IP) of IRAK4 with MyD88 followed by western analysis of the proteins indicated. (D) Whole cell lysates (WCL) were collected and DCs were monitored for STAT-1 phosphorylation and viperin expression after treatment with LPS oxPAPC. (C and D) One experiment representative of three is shown.
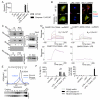
Fig. 2. oxPAPC induces the activation of the NLRP3 inflammasome in DCs
(A) DCs primed with LPS, followed by ATP or oxPAPC treatment. Cell culture supernatant from DCs subjected to indicated treatments were collected, and processed IL-1β (p17) production was assessed. One experiment representative of three is shown. (B) DCs were treated with LPS alone, 10, 50, or 120μM oxPAPC or were primed with LPS for 3 hours and then treated with oxPAPC. For this experiment, commercially available oxPAPC and an oxPAPC enriched in PEIPC were used. 18 hours after LPS administration, secreted (left panel) and cell associated (right panel) IL-1β were measured by ELISA. Means and standard deviations of four replicates are shown. (C) DCs of the genotypes indicated were treated with LPS alone, oxPAPC alone or were primed with LPS for 3 hours and then treated with oxPAPC. 18 hours after LPS administration, IL-1β and secretion was measured by ELISA. Means and standard deviations of four replicates are shown. (D) MΦs and DCs were treated with Pam3CSK (P3C) alone, oxPAPC alone, or were primed with Pam3CSK for 3 hours and then treated with oxPAPC, DOTAP alone, and LPS or oxPAPC encapsulated in DOTAP. 18 hours after P3C administration, IL-1β was measured by ELISA. Means and standard deviations of four replicates are shown. (E) DCs (left panel) or MΦs (right panel) were primed with LPS for three hours and treated with ATP. At indicated time points, IL-1β was measured by ELISA and cell death was measured by PI permeabilization assay. Means and standard deviations of four replicates are shown.
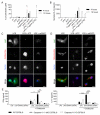
Fig. 3. oxPAPC promotes noncanonical inflammasome activation
(A) WT DC and caspase-11 KO DC were treated with LPS alone, oxPAPC alone or were primed with LPS for 3 hours and then treated with oxPAPC. 18 hours after LPS administration, IL-1β secretion was measured by ELISA. Means and standard deviations of four replicates are shown. (B) DCs were left untreated or primed with LPS and then stimulated with ATP or oxPAPC. Specks containing ASC (green) and caspase-1 (Casp1, red) were analyzed 18 hours after LPS stimulation. Nuclei are shown in blue. Panels are representative of four independent experiments. Scale bar: 10 μm. (C) S100 fractions of nontreated (nt) or P3C-primed (P3C) MΦs were incubated with biotin-LPS (Bio-LPS), biotin-oxPAPC (Bio-oxPAPC) or biotin-MDP (Bio-MDP). Endogenous proteins associated with biotinylated-ligands were captured by streptavidin beads and revealed by western analysis. Shown is a representative blot out of three independent experiments. (D) SPR analysis of the interactions between the proteins and lipids indicated. (E) Gel filtration analysis of the size of caspase-11 complexes before and after exposure to oxPAPC. Complex size was monitored by A280 or western analysis, as indicated. Shown is a representative blot out of three independent experiments. (F) Bone marrow cells were infected with the pMSCV2.2-IRES-GFP vector (empty), the pMSCV2.2-IRES-GFP vector encoding WT caspase-11 (WT caspase-11) or the same vector containing a catalytic mutant caspase-11 (C254A). DCs were primed or not with LPS and then stimulated with oxPAPC, or transfected with LPS-containing FuGENE (Trans. LPS). 18 hours after LPS priming, supernatant were collected and IL-1β was measured by ELISA. Cell viability was assessed by measuring LDH release. Means and standard deviations of four replicates are shown.
Fig. 4. oxPAPC prevents DC death and potentiates adaptive immune responses
(A and B) DCs were treated with LPS alone, ATP alone, oxPAPC alone or FuGENE complexed LPS [Fugene (LPS)], or were primed for three hours with LPS and then treated with the indicated stimuli. Cell death was measured by LDH release (A) or IL-1β secretion was measured by ELISA (B). Means and standard deviations of four replicates are shown. (C and D) DCs were pretreated with LPS for 3 hours and then activated with ATP or oxPAPC. 18 hours later, cells were stained for ASC (green), nuclei (blue) Zombie Dye (red) (C) or active mitochondria (red). Scale bar: 10 μm. (D). Panels are representative of three independent experiments. (E) CD4+ T-cells were isolated from the draining lymph nodes 40 days after immunization with OVA + LPS in IFA (LPS), OVA + LPS + oxPAPC in IFA (LPS+oxPAPC) or OVA + oxPAPC in IFA (oxPAPC) of WT, caspase-1/-11 dKO or caspase-11 KO mice. CD4+ T-cells were restimulated or not with OVA in the presence of DCs. IFNγ (left panel) and IL-17 (right panel) secretion was measured 5 days later by ELISA. Bar graphs represent means and standard errors of two experiments with five animals per group. *P < 0.05; **P < 0.01; ***P < 0.005.
Comment in
- IMMUNOLOGY. A lipid arsenal to control inflammation.
Napier BA, Monack DM. Napier BA, et al. Science. 2016 Jun 3;352(6290):1173-4. doi: 10.1126/science.aag0366. Epub 2016 Jun 2. Science. 2016. PMID: 27257241 No abstract available. - Inflammasome: To die or not to die.
Minton K. Minton K. Nat Rev Immunol. 2016 Jul;16(7):404-5. doi: 10.1038/nri.2016.73. Epub 2016 Jun 20. Nat Rev Immunol. 2016. PMID: 27320315 No abstract available.
Similar articles
- IMMUNOLOGY. A lipid arsenal to control inflammation.
Napier BA, Monack DM. Napier BA, et al. Science. 2016 Jun 3;352(6290):1173-4. doi: 10.1126/science.aag0366. Epub 2016 Jun 2. Science. 2016. PMID: 27257241 No abstract available. - The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages.
Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM, Dorfleutner A, Stehlik C. Chu LH, et al. Nat Commun. 2018 Mar 8;9(1):996. doi: 10.1038/s41467-018-03409-3. Nat Commun. 2018. PMID: 29520027 Free PMC article. - Caspase-11 activation in response to bacterial secretion systems that access the host cytosol.
Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, Shin S. Casson CN, et al. PLoS Pathog. 2013;9(6):e1003400. doi: 10.1371/journal.ppat.1003400. Epub 2013 Jun 6. PLoS Pathog. 2013. PMID: 23762026 Free PMC article. - Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways.
Zamyatina A, Heine H. Zamyatina A, et al. Front Immunol. 2020 Nov 27;11:585146. doi: 10.3389/fimmu.2020.585146. eCollection 2020. Front Immunol. 2020. PMID: 33329561 Free PMC article. Review. - Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.
de Lima JD, de Paula AGP, Yuasa BS, de Souza Smanioto CC, da Cruz Silva MC, Dos Santos PI, Prado KB, Winter Boldt AB, Braga TT. de Lima JD, et al. Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
- Plexin C1 influences immune response to intracellular LPS and survival in murine sepsis.
Bernard A, Eggstein C, Tang L, Keller M, Körner A, Mirakaj V, Rosenberger P. Bernard A, et al. J Biomed Sci. 2024 Aug 21;31(1):82. doi: 10.1186/s12929-024-01074-x. J Biomed Sci. 2024. PMID: 39169397 Free PMC article. - Intestinal edema induced by LPS-induced endotoxemia is associated with an inflammasome adaptor ASC.
Yamamoto T, Kurata M, Kaneko N, Masumoto J. Yamamoto T, et al. PLoS One. 2023 Feb 17;18(2):e0281746. doi: 10.1371/journal.pone.0281746. eCollection 2023. PLoS One. 2023. PMID: 36800329 Free PMC article. - Evolutionary analyses of the gasdermin family suggest conserved roles in infection response despite loss of pore-forming functionality.
Angosto-Bazarra D, Alarcón-Vila C, Hurtado-Navarro L, Baños MC, Rivers-Auty J, Pelegrín P. Angosto-Bazarra D, et al. BMC Biol. 2022 Jan 7;20(1):9. doi: 10.1186/s12915-021-01220-z. BMC Biol. 2022. PMID: 34996441 Free PMC article. - Toll-Like Receptor 4 Mediates Methamphetamine-Induced Neuroinflammation through Caspase-11 Signaling Pathway in Astrocytes.
Du SH, Qiao DF, Chen CX, Chen S, Liu C, Lin Z, Wang H, Xie WB. Du SH, et al. Front Mol Neurosci. 2017 Dec 12;10:409. doi: 10.3389/fnmol.2017.00409. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29311802 Free PMC article. - Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation.
Yotsumoto S, Muroi Y, Chiba T, Ohmura R, Yoneyama M, Magarisawa M, Dodo K, Terayama N, Sodeoka M, Aoyagi R, Arita M, Arakawa S, Shimizu S, Tanaka M. Yotsumoto S, et al. Sci Rep. 2017 Nov 22;7(1):16026. doi: 10.1038/s41598-017-15668-z. Sci Rep. 2017. PMID: 29167447 Free PMC article.
References
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front. Immunol. 2012;3:287. Medline doi:10.3389/fimmu.2012.00287. - DOI - PMC - PubMed
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. Medline doi:10.1038/nri2215. - DOI - PMC - PubMed
- Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. Medline doi:10.1084/jem.20031763. - DOI - PMC - PubMed
- Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N. Engl. J. Med. 2005;353:9–11. Medline doi:10.1056/NEJMp058118. - DOI - PubMed
- Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr. Opin. Lipidol. 2003;14:421–430. Medline doi:10.1097/00041433-200310000-00002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI093589/AI/NIAID NIH HHS/United States
- R01 AI121066/AI/NIAID NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- K99 AI072955/AI/NIAID NIH HHS/United States
- P30 DK34854/DK/NIDDK NIH HHS/United States
- 1R01AI121066-01A1/AI/NIAID NIH HHS/United States
- 1R15HL121770-01A1/HL/NHLBI NIH HHS/United States
- R15 HL121770/HL/NHLBI NIH HHS/United States
- AI072955/AI/NIAID NIH HHS/United States
- R56 AI093589/AI/NIAID NIH HHS/United States
- AI093589/AI/NIAID NIH HHS/United States
- R00 AI072955/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous