Microglia across the lifespan: from origin to function in brain development, plasticity and cognition - PubMed (original) (raw)
Review
. 2017 Mar 15;595(6):1929-1945.
doi: 10.1113/JP272134. Epub 2016 May 29.
Affiliations
- PMID: 27104646
- PMCID: PMC5350449
- DOI: 10.1113/JP272134
Review
Microglia across the lifespan: from origin to function in brain development, plasticity and cognition
Tuan Leng Tay et al. J Physiol. 2017.
Abstract
Microglia are the only immune cells that permanently reside in the central nervous system (CNS) alongside neurons and other types of glial cells. The past decade has witnessed a revolution in our understanding of their roles during normal physiological conditions. Cutting-edge techniques revealed that these resident immune cells are critical for proper brain development, actively maintain health in the mature brain, and rapidly adapt their function to physiological or pathophysiological needs. In this review, we highlight recent studies on microglial origin (from the embryonic yolk sac) and the factors regulating their differentiation and homeostasis upon brain invasion. Elegant experiments tracking microglia in the CNS allowed studies of their unique roles compared with other types of resident macrophages. Here we review the emerging roles of microglia in brain development, plasticity and cognition, and discuss the implications of the depletion or dysfunction of microglia for our understanding of disease pathogenesis. Immune activation, inflammation and various other conditions resulting in undesirable microglial activity at different stages of life could severely impair learning, memory and other essential cognitive functions. The diversity of microglial phenotypes across the lifespan, between compartments of the CNS, and sexes, as well as their crosstalk with the body and external environment, is also emphasised. Understanding what defines particular microglial phenotypes is of major importance for future development of innovative therapies controlling their effector functions, with consequences for cognition across chronic stress, ageing, neuropsychiatric and neurological diseases.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
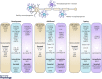
Figure 1. Signalling pathways regulating microglial proliferation, maturation and homeostasis in healthy and diseased conditions
The proper development, differentiation, proliferation and function of both microglia and neurons require a continuous and lifelong crosstalk between the two cell types via complementary ligands and receptors and secreted trophic factors (e.g. cytokines). As microglia (blue) and neurons (brown) mature, they downregulate certain signalling pathways (e.g. Cxcl12‐Cxcr4) and upregulate others (e.g. IL‐34‐Csf‐1R). Dysregulation of either ligands or receptors occurs in a number of disease processes (dysfunctional microglia, purple). Maturation of yolk sac derived microglia within the CNS is dependent on proper signalling via purinergic receptors and cell surface protein Csf‐1R. Similarly, mature microglia require functional Csf‐1R signalling for their maintenance. Microglial ‘signature’ factors recently identified include TGFβ (transforming growth factor β, which appears critical for mediating microglial survival and phenotypic differentiation in the healthy mature brain) and Tmem119 (transmembrane protein 119, a cell surface protein of unknown function). The expression of microglial markers (including IBA1, CD45, MHC class II, and others) is often changed during the course of ageing and disease, coincident with changes in microglial function. R, receptors.
Similar articles
- Physiology of Microglia.
Tay TL, Carrier M, Tremblay MÈ. Tay TL, et al. Adv Exp Med Biol. 2019;1175:129-148. doi: 10.1007/978-981-13-9913-8_6. Adv Exp Med Biol. 2019. PMID: 31583587 Review. - Microglia in steady state.
Kierdorf K, Prinz M. Kierdorf K, et al. J Clin Invest. 2017 Sep 1;127(9):3201-3209. doi: 10.1172/JCI90602. Epub 2017 Jul 17. J Clin Invest. 2017. PMID: 28714861 Free PMC article. Review. - What Do Microglia Really Do in Healthy Adult Brain?
Augusto-Oliveira M, Arrifano GP, Lopes-Araújo A, Santos-Sacramento L, Takeda PY, Anthony DC, Malva JO, Crespo-Lopez ME. Augusto-Oliveira M, et al. Cells. 2019 Oct 22;8(10):1293. doi: 10.3390/cells8101293. Cells. 2019. PMID: 31652490 Free PMC article. Review. - Microglia heterogeneity along a spatio-temporal axis: More questions than answers.
Silvin A, Ginhoux F. Silvin A, et al. Glia. 2018 Oct;66(10):2045-2057. doi: 10.1002/glia.23458. Epub 2018 Aug 25. Glia. 2018. PMID: 30144321 Review. - Microglia in CNS development: Shaping the brain for the future.
Mosser CA, Baptista S, Arnoux I, Audinat E. Mosser CA, et al. Prog Neurobiol. 2017 Feb-Mar;149-150:1-20. doi: 10.1016/j.pneurobio.2017.01.002. Epub 2017 Jan 28. Prog Neurobiol. 2017. PMID: 28143732 Review.
Cited by
- The arrangements of the microvasculature and surrounding glial cells are linked to blood-brain barrier formation in the cerebral cortex.
Shigemoto-Mogami Y, Nakayama-Kitamura K, Sato K. Shigemoto-Mogami Y, et al. Front Neuroanat. 2024 Aug 7;18:1438190. doi: 10.3389/fnana.2024.1438190. eCollection 2024. Front Neuroanat. 2024. PMID: 39170850 Free PMC article. - Macrophages and Stem Cells-Two to Tango for Tissue Repair?
Manole E, Niculite C, Lambrescu IM, Gaina G, Ioghen O, Ceafalan LC, Hinescu ME. Manole E, et al. Biomolecules. 2021 May 6;11(5):697. doi: 10.3390/biom11050697. Biomolecules. 2021. PMID: 34066618 Free PMC article. Review. - Interleukin 4 Affects Epilepsy by Regulating Glial Cells: Potential and Possible Mechanism.
Chen L, Zhu L, Lu D, Wu Z, Han Y, Xu P, Chang L, Wu Q. Chen L, et al. Front Mol Neurosci. 2020 Sep 4;13:554547. doi: 10.3389/fnmol.2020.554547. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33013320 Free PMC article. Review. - Benefits of bone marrow mesenchymal stem cells compared to their conditioned medium in valproic acid-induced autism in rats.
Noshadian M, Ragerdi Kashani I, Asadi-Golshan R, Zarini D, Ghafari N, Zahedi E, Pasbakhsh P. Noshadian M, et al. Mol Biol Rep. 2024 Feb 24;51(1):353. doi: 10.1007/s11033-024-09292-0. Mol Biol Rep. 2024. PMID: 38401030 - Pharmacological Tools to Activate Microglia and their Possible use to Study Neural Network Patho-physiology.
Pena-Ortega F. Pena-Ortega F. Curr Neuropharmacol. 2017;15(4):595-619. doi: 10.2174/1570159X14666160928151546. Curr Neuropharmacol. 2017. PMID: 27697040 Free PMC article. Review.
References
- Abutbul S, Shapiro J, Szaingurten‐Solodkin I, Levy N, Carmy Y, Baron R, Jung S & Monsonego A (2012). TGF‐beta signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60, 1160–1171. - PubMed
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B, Martino G & Muzio L (2014). Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun 5, 5611. - PubMed
- Arnoux I, Hoshiko M, Mandavy L, Avignone E, Yamamoto N & Audinat E (2013). Adaptive phenotype of microglial cells during the normal postnatal development of the somatosensory “Barrel” cortex. Glia 61, 1582–1594. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous