The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes - PubMed (original) (raw)
. 2016 Jun 2;44(10):4551-64.
doi: 10.1093/nar/gkw322. Epub 2016 Apr 25.
Affiliations
- PMID: 27112572
- PMCID: PMC4889955
- DOI: 10.1093/nar/gkw322
The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes
Darius Kazlauskas et al. Nucleic Acids Res. 2016.
Abstract
Genomic DNA replication is a complex process that involves multiple proteins. Cellular DNA replication systems are broadly classified into only two types, bacterial and archaeo-eukaryotic. In contrast, double-stranded (ds) DNA viruses feature a much broader diversity of DNA replication machineries. Viruses differ greatly in both completeness and composition of their sets of DNA replication proteins. In this study, we explored whether there are common patterns underlying this extreme diversity. We identified and analyzed all major functional groups of DNA replication proteins in all available proteomes of dsDNA viruses. Our results show that some proteins are common to viruses infecting all domains of life and likely represent components of the ancestral core set. These include B-family polymerases, SF3 helicases, archaeo-eukaryotic primases, clamps and clamp loaders of the archaeo-eukaryotic type, RNase H and ATP-dependent DNA ligases. We also discovered a clear correlation between genome size and self-sufficiency of viral DNA replication, the unanticipated dominance of replicative helicases and pervasive functional associations among certain groups of DNA replication proteins. Altogether, our results provide a comprehensive view on the diversity and evolution of replication systems in the DNA virome and uncover fundamental principles underlying the orchestration of viral DNA replication.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
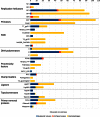
Figure 1.
Quantitative distribution of DNA replication protein families and the taxonomy of virus host. The length of the bar is proportional to the number of protein family members found in the representative set of dsDNA viruses. Asterisk (*) denotes herpes processivity factors that include UL42, UL44 and BMRF1 homologs.
Figure 2.
Diversity of replicative helicases domain organizations in dsDNA viruses. The number of representative sequences having specific domain organization is indicated on the left. The catalytic domain of the helicase is shown in green; primase, orange; polymerase, red; other domains, gray. αHD, α-helical domain; DUF, domain of unknown function; WH, winged-helix-turn-helix domain; ZnBD, Zn-binding domain; DBD, DNA-binding domain; Pol, DNA polymerase.
Figure 3.
Replication proteins arranged by their overall observed frequencies (%) in genomes of dsDNA viruses.
Figure 4.
Maximum likelihood phylogenetic analysis of family B DNA polymerases from archaea, bacteria, eukaryotes and their respective viruses. Numbers at the branch points represent non-parametric bootstrap support (1000 replicates). Branches are color-coded and the color key is provided at the bottom right corner of the figure. Alpha, Delta, Epsilon and Zeta represent subclasses of eukaryotic PolBs. ‘N’ indicates that only the catalytic N-terminal domain of the PolB-Epsilon was considered. The scale bar represents the number of substitutions per site.
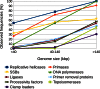
Figure 5.
Relationship between the observed frequencies of viral DNA replication proteins and the genome size of dsDNA viruses.
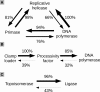
Figure 6.
Associations between DNA replication proteins based on their presence/absence in dsDNA virus genomes. An arrow pointing from the protein indicates the percent of cases when this protein is encoded together with the protein at the tip of the arrow.
Similar articles
- Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: structural insights and new members.
Iyer LM, Koonin EV, Leipe DD, Aravind L. Iyer LM, et al. Nucleic Acids Res. 2005 Jul 15;33(12):3875-96. doi: 10.1093/nar/gki702. Print 2005. Nucleic Acids Res. 2005. PMID: 16027112 Free PMC article. - The Polyphyletic Origins of Primase-Helicase Bifunctional Proteins.
Gupta A, Patil S, Vijayakumar R, Kondabagil K. Gupta A, et al. J Mol Evol. 2017 Dec;85(5-6):188-204. doi: 10.1007/s00239-017-9816-6. Epub 2017 Nov 15. J Mol Evol. 2017. PMID: 29143083 - Multiple occurrences of giant virus core genes acquired by eukaryotic genomes: the visible part of the iceberg?
Filée J. Filée J. Virology. 2014 Oct;466-467:53-9. doi: 10.1016/j.virol.2014.06.004. Epub 2014 Jul 4. Virology. 2014. PMID: 24998348 - Evolutionary genomics of nucleo-cytoplasmic large DNA viruses.
Iyer LM, Balaji S, Koonin EV, Aravind L. Iyer LM, et al. Virus Res. 2006 Apr;117(1):156-84. doi: 10.1016/j.virusres.2006.01.009. Epub 2006 Feb 21. Virus Res. 2006. PMID: 16494962 Review. - Single-stranded DNA viruses employ a variety of mechanisms for integration into host genomes.
Krupovic M, Forterre P. Krupovic M, et al. Ann N Y Acad Sci. 2015 Apr;1341:41-53. doi: 10.1111/nyas.12675. Epub 2015 Feb 11. Ann N Y Acad Sci. 2015. PMID: 25675979 Review.
Cited by
- Multiple origins of prokaryotic and eukaryotic single-stranded DNA viruses from bacterial and archaeal plasmids.
Kazlauskas D, Varsani A, Koonin EV, Krupovic M. Kazlauskas D, et al. Nat Commun. 2019 Jul 31;10(1):3425. doi: 10.1038/s41467-019-11433-0. Nat Commun. 2019. PMID: 31366885 Free PMC article. - Two-Metal Ion-Dependent Enzymes as Potential Antiviral Targets in Human Herpesviruses.
DiScipio KA, Weerasooriya S, Szczepaniak R, Hazeen A, Wright LR, Wright DL, Weller SK. DiScipio KA, et al. mBio. 2022 Feb 22;13(1):e0322621. doi: 10.1128/mbio.03226-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073739 Free PMC article. - Multiple origins of viral capsid proteins from cellular ancestors.
Krupovic M, Koonin EV. Krupovic M, et al. Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):E2401-E2410. doi: 10.1073/pnas.1621061114. Epub 2017 Mar 6. Proc Natl Acad Sci U S A. 2017. PMID: 28265094 Free PMC article. - Molecular Analysis of Arthrobacter Myovirus vB_ArtM-ArV1: We Blame It on the Tail.
Kaliniene L, Šimoliūnas E, Truncaitė L, Zajančkauskaitė A, Nainys J, Kaupinis A, Valius M, Meškys R. Kaliniene L, et al. J Virol. 2017 Mar 29;91(8):e00023-17. doi: 10.1128/JVI.00023-17. Print 2017 Apr 15. J Virol. 2017. PMID: 28122988 Free PMC article. - Conserved Secondary Structures in Viral mRNAs.
Kiening M, Ochsenreiter R, Hellinger HJ, Rattei T, Hofacker I, Frishman D. Kiening M, et al. Viruses. 2019 Apr 29;11(5):401. doi: 10.3390/v11050401. Viruses. 2019. PMID: 31035717 Free PMC article.
References
- Forterre P., Prangishvili D. The major role of viruses in cellular evolution: facts and hypotheses. Curr. Opin. Virol. 2013;3:558–565. - PubMed
- Suttle C.A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol. 2007;5:801–812. - PubMed
- Kornberg A., Baker T.A. Dna Replication. Sausalito: University ScienceBooks; 2005.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources