Monocyte Trafficking, Engraftment, and Delivery of Nanoparticles and an Exogenous Gene into the Acutely Inflamed Brain Tissue - Evaluations on Monocyte-Based Delivery System for the Central Nervous System - PubMed (original) (raw)
Monocyte Trafficking, Engraftment, and Delivery of Nanoparticles and an Exogenous Gene into the Acutely Inflamed Brain Tissue - Evaluations on Monocyte-Based Delivery System for the Central Nervous System
Hsin-I Tong et al. PLoS One. 2016.
Abstract
The ability of monocytes and monocyte-derived macrophages (MDM) to travel towards chemotactic gradient, traverse tissue barriers, and accumulate precisely at diseased sites makes them attractive candidates as drug carriers and therapeutic gene delivery vehicles targeting the brain, where treatments are often hampered by the blockade of the blood brain barrier (BBB). This study was designed to fully establish an optimized cell-based delivery system using monocytes and MDM, by evaluating their homing efficiency, engraftment potential, as well as carriage and delivery ability to transport nano-scaled particles and exogenous genes into the brain, following the non-invasive intravenous (IV) cell adoptive transfer in an acute neuroinflammation mouse model induced by intracranial injection of Escherichia coli lipopolysaccharides. We demonstrated that freshly isolated monocytes had superior inflamed-brain homing ability over MDM cultured in the presence of macrophage colony stimulating factor. In addition, brain trafficking of IV infused monocytes was positively correlated with the number of adoptive transferred cells, and could be further enhanced by transient disruption of the BBB with IV administration of Mannitol, Bradykinin or Serotonin right before cell infusion. A small portion of transmigrated cells was detected to differentiate into IBA-1 positive cells with microglia morphology in the brain. Finally, with the use of superparamagnetic iron oxide nanoparticles SHP30, the ability of nanoscale agent-carriage monocytes to enter the inflamed brain region was validated. In addition, lentiviral vector DHIV-101 was used to introduce green fluorescent protein (GFP) gene into monocytes, and the exogenous GFP gene was detected in the brain at 48 hours following IV infusion of the transduced monocytes. All together, our study has set up the optimized conditions for the more-in-depth tests and development of monocyte-mediated delivery, and our data supported the notion to use monocytes as a non-invasive cell-based delivery system for the brain.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
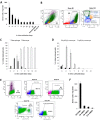
Fig 1. Migration efficiency of IV transferred monocytes and cMDM to acutely inflamed brain tissue is linked to monocyte-to-macrophage differentiation.
(A) MDM cultured from day 0 to day 28 were introduced IV (5×106 cells) into recipient mice bearing LPS-induced acute neuroinflammaiton, and cMDM in vivo brain homing efficiency deceased as the in vitro cultivation time went up. Female gDNA = female mouse brain tissue genomic DNA (negative control). No cell IV = LPS injected control animal that received no cell IV transplant. Final data presented here represents mean values ± SD. Data was analyzed by one-way ANOVA, with resulting p-value <0.05. (B–D) Flow cytometry analysis on cMDM cell populations at different time points post in vitro cultivation. (B) Example of phenotype identification of cMDM using D5 cMDM. The green cluster reflects macrophage phenotype (CD11b+ F4/80hi Ly6Clow to neg), blue cluster reflects Ly6clow monocytes (CD11b+ F4/80low to med Ly6Clow to neg), and yellow cluster reflects Ly6Chi monocytes (CD11b+ F4/80low to med Ly6Chigh). (C) The ratio of macrophages (CD11b+ F4/80high Ly6Cneg) and total monocytes (CD11b+ F4/80low to med Ly6Clow to high), and (C) the ratio of Ly6Chi monocytes (CD11b+ F4/80med Ly6Chigh CCR2+) and Ly6Clo monocytes (CD11b+ F4/80low Ly6Clow CCR2low to neg) in culture were evaluated. (E) Phenotyping of freshly isolated EnMO by flow cytometry analysis. Expression level of CD115, CD11b, F4/80, and Ly6c were measured to determine the purity of the enriched cells. (F) Freshly isolated EnMO showed superior brain homing efficiency over cMDM. 5x106 of EnMO (D0 EnMO), or MDM cultured for 2-, 5-, or 12- days (D2 cMDM, D5 cMDM, D12 cMDM, respectively) were infused IV to animals with acute neuroinlfammation, and the number of donor cells present in the LPS-injected brain hemisphere was quantified at 48 hour following cell IVI. Final data presented here represents mean values ± SD. Data was analyzed by one-way ANOVA, with resulting p-value all < 0.05.
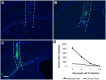
Fig 2. IV transferred monocytes ingression in brain.
(A-C) Representative sections show the distribution of GFP positive donor-derived monocytes in the LPS injected brain at day 2(A), day 5(B), and day 7(C) post monocytes IV transfer. The area between the two white dash lines indicated the physical needle insertion site. For panel A-C, bar represent 200 μm, original magnification ×40. (D) Number of donor cells detected in the LPS injected hemisphere (ipsi hemi = ipsilateral hemi) and in the other hemisphere (contral hemi = contralateral) decreased over time post monocyte IV transfer.
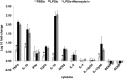
Fig 3. Expression analysis of selected cytokine genes in inflamed brain region.
Transcription of TNFα, IL-1β, TGFβ, IL-10, IL-12p40 and NOS2 were significantly different in group PBSic compared to group LPSic and group LPSic+Monocytes iv (p-value < 0.05), but no difference was observed between group LPSic and group LPSic+Monocyte iv, indicating the presence of additional IV infused monocytes in circulation did not aggravate neuroinflammation. PBSic = sham control in which animals received PBS ICI. LPSic = inflammation control in which animals received LPS ICI. LPSic + Monocyte iv = animals received LPS ICI 24 hours prior to monocytes IV transfer. Cytokine gene transcription levels were measured at 72 hours post ICI. * = p-value < 0.05, unpaired t test. Final data presented here represents mean values +/- SD.
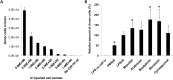
Fig 4. Enhanced monocytes entry into the inflamed brain.
The amount of IV infused monocytes recruited to the inflamed brain regions could be enhanced by (A) increasing IV transferred monocyte amounts, and (B) transiently disrupting BBB by chemical agents. (A) The number of recruited donor-derived monocytes in the brain was positively correlated to the number of IV infused cells, as analyzed by Pearson correlation coefficient, with R = 0.9932, and R2 = 0.9864. No Cell IVI control = Control animal that received LPS ICI but no monocyte IV. (B) Mannitol, Bradykinin, and Serotonin enhanced entry of IV transferred monocytes into LPS-inflamed brain tissue by 134%± 29%, 176%± 51%, and 168± 59% compared to the group that received no BBBD reagents (LPSctl, p value < 0.05, unpaired _t_ test). Two additional control groups were included: LPS no cell i.v. = mice that received LPS ICI but no cells, and PBSctl = mice that received PBS ICI, followed by monocyte IV transfer. Data was analyzed by unpaired _t_ test (to LPS no cell i.v. control) and one-way ANOVA (among all test groups), with resulting p-value <0.05 (*) deemed as significant. No difference was found in groups treated with Arabinose and Cyclosporin A (p value > 0.05, unpaired t test). Final data presented here represents mean values ± SD.
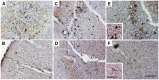
Fig 5. Recruited monocytes matured into cells with two distinguish morphologies in the brain.
Two types of GFP positive donor-derived monocytes with very distinct morphologies were identified in the inflamed brain at day 2 (A& B), day 5 (C& D), and day 7 (E& F) post monocytes IVI. Panels A, C, and E show cells with big and round morphology that were often detected along the LPS injection tract, while panels B, D, and F show microglia-like, highly branched cells that were usually found in the cortical regions, further away from the needle tract. GFP positive cells were visualized by IHC staining with a GFP-specific primary antibody and a biotin-conjugated secondary antibody; Bars represent 100 μm (A-F) and 25 μm (insets). Original magnification ×100 for panel A-F.
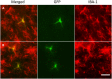
Fig 6. Recruited monocytes differentiated into IBA-1 positive cells with microglia morphology in the brain.
GFP positive donor-derived cells (green) with a highly branched, microglia-like morphology located throughout the cortical region were Iba-1 positive (red) by day 5 (A) and day 7 (B) following IV adoptive transfer of monocytes. Bar represent 25 μm for all panel. Original magnification ×200 for panel A-F.
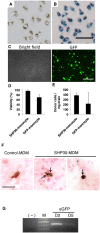
Fig 7. Monocyte-mediated delivery of SHP30 and GFP gene to the brain.
(A&B) Overnight incubation of SHP30 with monocytes resulted in 100% SHP30 uptake efficiency, as observed by (A) the presence of brown SHP30 accumulates under the light microscopic fields, and by (B) the presence of Prussian blue pigments at monocytes cytoplasm following Prussian blue staining. (C) D101 LV-mediated GFP gene transfer resulted in around 36% transduction efficiency. (D) Viability of ex vivo modified monocytes following overnight incubation with 25 μg/mL SHP30 (SHP30-monocyte), and 90-minute transduction with D101 LV (GFP-monocyte). (E) SHP30-laden monocytes (SHP30-monocyte) or monocytes underwent D101 LV transduction (GFP-monocyte) were detected in the inflamed brain regions at 48 hours following cell IV infusion. (F) The presence of SHP30 (Dark blue, Prussian blue positive) was detected in the cytoplasm of the recruited donor-derived cells (Brown, GFP positive) in the brain. The arrow showed the presence of SHP30. (G) The presence of the exogenous GFP gene was detected in the inflamed brain by PCR at day 2 and day 5 following cell IV transfer. For Panel A &B, bar represents 50 μm, at original maginications x200. For panel C, bar represents 200 μm, at original magnification ×200. For panel F, bar represents 25 μm, at original magnification x400.
Similar articles
- Evaluation on Monocyte-Mediated Delivery of a Therapeutic Gene into the Inflamed Brain.
Tong HI, Kang W, Zhou G, Liu M, Shi Y, Lu Y. Tong HI, et al. Curr Gene Ther. 2017;16(6):401-409. doi: 10.2174/1566523217666161118165710. Curr Gene Ther. 2017. PMID: 27903224 - GFP-lentiviral vectors targeting for neuroAIDS.
Lu Y. Lu Y. Methods Mol Biol. 2009;515:177-97. doi: 10.1007/978-1-59745-559-6_12. Methods Mol Biol. 2009. PMID: 19378133 - Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo.
Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DE, Stylianou E, McShane H, Channon KM, Chawla A, Greaves DR. Iqbal AJ, et al. Blood. 2014 Oct 9;124(15):e33-44. doi: 10.1182/blood-2014-04-568691. Epub 2014 Jul 16. Blood. 2014. PMID: 25030063 Free PMC article. - Nano carriers for drug transport across the blood-brain barrier.
Li X, Tsibouklis J, Weng T, Zhang B, Yin G, Feng G, Cui Y, Savina IN, Mikhalovska LI, Sandeman SR, Howel CA, Mikhalovsky SV. Li X, et al. J Drug Target. 2017 Jan;25(1):17-28. doi: 10.1080/1061186X.2016.1184272. Epub 2016 May 19. J Drug Target. 2017. PMID: 27126681 Review.
Cited by
- Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair.
Larson TA. Larson TA. Front Endocrinol (Lausanne). 2018 Apr 30;9:205. doi: 10.3389/fendo.2018.00205. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29760681 Free PMC article. Review. - Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles.
Konopka CJ, Wozniak M, Hedhli J, Ploska A, Schwartz-Duval A, Siekierzycka A, Pan D, Munirathinam G, Dobrucki IT, Kalinowski L, Dobrucki LW. Konopka CJ, et al. Theranostics. 2018 Oct 5;8(18):5012-5024. doi: 10.7150/thno.24791. eCollection 2018. Theranostics. 2018. PMID: 30429883 Free PMC article. - Neuroinflammation Treatment via Targeted Delivery of Nanoparticles.
Cerqueira SR, Ayad NG, Lee JK. Cerqueira SR, et al. Front Cell Neurosci. 2020 Sep 30;14:576037. doi: 10.3389/fncel.2020.576037. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192321 Free PMC article. Review. - Nanoparticle Interactions with the Blood Brain Barrier: Insights from Drosophila and Implications for Human Astrocyte Targeted Therapies.
Padti AC, Bhavi SM, Thokchom B, Singh SR, Bhat SS, Harini BP, Sillanpää M, Yarajarla RB. Padti AC, et al. Neurochem Res. 2025 Jan 20;50(1):80. doi: 10.1007/s11064-025-04333-x. Neurochem Res. 2025. PMID: 39832031 Review. - Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype.
Wofford KL, Singh BS, Cullen DK, Spiller KL. Wofford KL, et al. Acta Biomater. 2020 Jan 1;101:237-248. doi: 10.1016/j.actbio.2019.11.021. Epub 2019 Nov 13. Acta Biomater. 2020. PMID: 31731024 Free PMC article.
References
- Burke B, Lewis CE. The macrophage. 2nd ed Oxford: Oxford University Press; 2002. xxvii, 647 p. p.
- Lech M, Grobmayr R, Weidenbusch M, Anders HJ. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediators Inflamm. 2012;2012:951390 Epub 2012/12/20. 10.1155/2012/951390 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical