Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction - PubMed (original) (raw)
. 2016 Jun 10;118(12):1918-1929.
doi: 10.1161/CIRCRESAHA.116.308688. Epub 2016 Apr 28.
Guangming Cheng # 2, Runming Jin # 1, Muhammad R Afzal 2, Anweshan Samanta 2, Yu-Ting Xuan 2, Magdy Girgis 2, Harold K Elias 3, Yanqing Zhu 2, Arash Davani 2, Yanjuan Yang 2, Xing Chen 2, Sheng Ye 2, Ou-Li Wang 2, Lei Chen 2, Jeryl Hauptman 2, Robert J Vincent 2, Buddhadeb Dawn 2
Affiliations
- PMID: 27126808
- PMCID: PMC4902783
- DOI: 10.1161/CIRCRESAHA.116.308688
Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction
Lin Zhao et al. Circ Res. 2016.
Erratum in
- Correction to: Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction.
[No authors listed] [No authors listed] Circ Res. 2020 Mar 27;126(7):e35. doi: 10.1161/RES.0000000000000325. Epub 2020 Mar 26. Circ Res. 2020. PMID: 32213131 No abstract available.
Abstract
Rationale: The role of interleukin (IL)-6 in the pathogenesis of cardiac myocyte hypertrophy remains controversial.
Objective: To conclusively determine whether IL-6 signaling is essential for the development of pressure overload-induced left ventricular (LV) hypertrophy and to elucidate the underlying molecular pathways.
Methods and results: Wild-type and IL-6 knockout (IL-6(-/-)) mice underwent sham surgery or transverse aortic constriction (TAC) to induce pressure overload. Serial echocardiograms and terminal hemodynamic studies revealed attenuated LV hypertrophy and superior preservation of LV function in IL-6(-/-) mice after TAC. The extents of LV remodeling, fibrosis, and apoptosis were reduced in IL-6(-/-) hearts after TAC. Transcriptional and protein assays of myocardial tissue identified Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) and signal transducer and activator of transcription 3 (STAT3) activation as important underlying mechanisms during cardiac hypertrophy induced by TAC. The involvement of these pathways in myocyte hypertrophy was verified in isolated cardiac myocytes from wild-type and IL-6(-/-) mice exposed to prohypertrophy agents. Furthermore, overexpression of CaMKII in H9c2 cells increased STAT3 phosphorylation, and exposure of H9c2 cells to IL-6 resulted in STAT3 activation that was attenuated by CaMKII inhibition. Together, these results identify the importance of CaMKII-dependent activation of STAT3 during cardiac myocyte hypertrophy via IL-6 signaling.
Conclusions: Genetic deletion of IL-6 attenuates TAC-induced LV hypertrophy and dysfunction, indicating a critical role played by IL-6 in the pathogenesis of LV hypertrophy in response to pressure overload. CaMKII plays an important role in IL-6-induced STAT3 activation and consequent cardiac myocyte hypertrophy. These findings may have significant therapeutic implications for LV hypertrophy and failure in patients with hypertension.
Keywords: Ca2+/calmodulin-dependent protein kinase type II; cardiac myocyte; hypertension; interleukin-6; left ventricular hypertrophy; signal transducer and activator of transcription 3.
© 2016 American Heart Association, Inc.
Figures
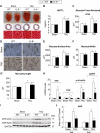
Figure 1. Genetic deletion of IL-6 attenuated LV hypertrophy induced by pressure overload in vivo
A, representative images showing gross cardiac morphology used for HW/TL calculation (upper panel); transverse sections stained with Masson's trichrome (middle panel); microscopic cross-sections stained with Masson's trichrome and used for calculation of transverse myocyte area (lower panel), scale bars, 50μm (n=10-12 per group). B-C, bar graphs showing quantitative data for HW/TL (B) and myocyte cross sectional area (C) (n=10-12 per group). D, representative images of isolated cardiac myocytes used for the assessment of myocyte cell surface area, myocyte width and myocyte length, scale bars, 100μm. E, Quantitative assessment of myocyte cell surface area calculated from isolated cardiac myocytes (n=6 per group). F, Myocyte width calculated from isolated cardiac myocytes (n=6 per group). G, Myocyte length calculated from isolated cardiac myocytes (n=6 per group). H, Transcriptional profiling of cardiac hypertrophy-related genes using qRT-PCR at 2 weeks after surgery (n=6 per group). I and J, representative western blots with quantification showing expression of ANP in WT and IL-6−/− mice at 2 and 6 weeks after surgery (n=6 per group). Data represent means ± SEM. *P<0.05 vs. Sham WT mice; § P<0.05 vs. Sham IL-6−/− mice; # P<0.05 vs. TAC WT mice.
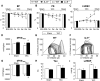
Figure 2. Genetic deletion of IL-6 prevented adverse remodeling induced by pressure overload
The parameters of LV remodeling were assessed using serial echocardiography and histological sections of harvested hearts arrested in diastole. A-D, serial echocardiographic quantitative data on LV mass (A), LV posterior wall end-diastolic thickness (B), LVEDD (C), and LVEDV (D); *P<0.05 vs. baseline (BSL) in TAC WT mice; § P<0.05 vs. BSL in TAC IL-6−/− mice; # P<0.05 vs TAC WT mice. E, myocardial sections from hearts arrested in end-diastole were used to calculate LV chamber size at 6 weeks after surgery. Data represent means ± SEM. *P<0.05 vs sham WT mice; # P<0.05 vs TAC WT mice. A-D, there was no statistically significant difference at baseline among Sham and TAC groups of WT and IL-6−/− mice.
Figure 3. Genetic deletion of IL-6 ameliorated cardiac dysfunction induced by pressure overload
A-C, LVEF, LVFS and LVESV were assessed by serial echocardiography in WT and IL-6−/− mice before and at 1, 2, 4 and 6 weeks after surgery (n=10-12 mice per group), *P<0.05 vs. BSL in TAC WT mice; § P<0.05 vs. BSL in TAC IL-6−/− mice, # P<0.05 vs. TAC WT mice. D, LV systolic function represented by LV contractility index (dP/dtmax) as assessed during invasive hemodynamics at 6 weeks after surgery (n=10-12 mice per group). E and F, representative LV pressure-volume loops from WT and IL-6−/− mice (loops from sham-operated animal are shown in black and those from TAC-operated in grey) at 6 weeks after surgery. ESPVR is represented by the line joining end-systolic pressure and volume coordinates. G and H, LV diastolic function represented by speed of cardiac relaxation (dP/dtmin) and relaxation constant (tau) as assessed by invasive hemodynamic studies at 6 weeks after surgery (n=10-12 mice per group). I, LV end-diastolic pressure (LVEDP) assessed during invasive hemodynamic study at 6 weeks after surgery (n=10-12 mice per group). *P<0.05 vs. Sham WT mice; # P<0.05 vs. TAC WT mice.
Figure 4. Genetic deletion of IL-6 attenuated pressure overload-induced LV fibrosis
Myocardial fibrosis was quantitated in picrosirius red-stained myocardial sections from WT and IL-6−/− mouse hearts at 6 weeks after surgery. A-D, representative images and quantification of interstitial (A and B) and perivascular fibrosis (C and D), scale bar, 50μm (n=9-14 mice per group). E, mRNA levels of Col1A1, Col3A1 and periostin were analyzed by qPCR from WT and IL-6−/− mouse hearts at 2 weeks after surgery. The relative abundance of transcripts were quantified and normalized to GAPDH (n=6 per group). F, representative Western immunoblots and quantitation of MMP9 and periostin protein levels from WT and IL-6−/− mouse hearts at 6 weeks after surgery (n=6 per group). Data represent means ± SEM. *P<0.05 vs. Sham WT mice; § P<0.05 vs. Sham IL-6−/− mice; # P<0.05 vs. TAC WT mice.
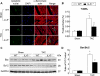
Figure 5. Attenuation of pressure overload-induced cardiac myocyte apoptosis in the absence of IL-6
A and B, TUNEL staining was performed on myocardial sections from WT and IL-6−/− hearts harvested at 6 weeks after surgery. Apoptotic nuclei (white arrows) are visualized by green fluorescence. Nuclei are identified in blue (DAPI). Sections were co-immunostained with anti-α-sarcomeric actin (red) to identify cardiac myocytes. TUNEL-positive myocyte nuclei were quantitated as a percentage of total cardiac myocyte nuclei, scale bar, 5μm (n=10-12 per group). C, representative Western immunoblots for Bax and Bcl-2. D, bar graph showing the ratio of Bax to Bcl-2 (n=6 per group). Data represent means ± SEM. *P<0.05 vs. Sham WT mice; # P<0.05 vs. TAC WT mice.
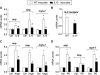
Figure 6. Requirement of IL-6 for adult cardiac myocyte hypertrophy
A, expression of hypertrophy-related genes (anp, bnp and myh-7) in adult mouse cardiac myocytes from WT and IL-6−/− mice following stimulation with rIL-6 (50ng/ml) for 24 h. B, expression of IL-6 receptor in WT and IL-6−/− cardiac myocytes. C, expression of anp, bnp and myh-7 in adult cardiac myocytes from WT and IL-6−/− mice following stimulation with Ang II (1×10−7 mol/L) for 48 h. D, expression of anp, bnp and myh-7 in adult cardiac myocytes from WT and IL-6−/− mice following stimulation with PE (10μmol/L) for 24 h. Data represent mean ± SEM from three independent experiments. *P<0.05 vs. WT cardiac myocytes; #P<0.05 vs. WT cardiac myocytes treated with pro-hypertrophic stimulators.
Figure 7. STAT3 and CaMKII contributed to IL-6-Induced cardiac myocyte hypertrophy
A-C, representative Western immunoblots (A) and quantitative data for pTyr-STAT3 (B) and p-CaMKII (C) in WT and IL-6−/− mouse hearts at 6 weeks after surgery (n=6 per group), *P<0.05 vs. Sham WT mice; #P<0.05 vs. TAC WT mice. D, representative images of H9c2 cells stained with 1% crystal violet after treatment with KN-62 (10μmol/L) or WP1066 (6μmol/L) in the presence and absence of rIL-6 (50ng/ml) for 24 h, scale bar=100 μm. E, cell surface area measured after 24 h of exposure to rIL-6 in the presence or absence of inhibitors of CaMKII or STAT3. F, Levels of mRNA for anp and bnp as determined by qPCR after 24 h of exposure to rIL-6 in the presence or absence of inhibitors of CaMKII and STAT3. (GI) stimulation with rIL-6 (50ng/ml) for variable duration with subsequent quantitation of pTyr-STAT3 (H) and p-CaMKII (I). Data represent means ± SEM from three independent experiments. *P<0.05 vs. control; # P<0.05 vs. rIL-6 treatment only.
Figure 8. IL-6-induced STAT3 phosphorylation and translocation in part via a CaMKII-dependent manner
A-C, H9c2 cells were transfected with 2, 4 and 6 μg of plasmids encoding CaMKII. Representative Western immunoblots (A) and quantitative data for CaMKII (B) and pTyr-STAT3 (C). D-F, representative Western immunoblots (D) and quantitative data for p-CaMKII (E) and pTyr-STAT3 (F) after stimulation with rIL-6 (50ng/ml) in the presence or absence of KN-62 (5μmol/L or 10μmol/L). G-I, representative Western immunoblots (G) and quantitative data for cytoplasmic and nuclear contents of p-CaMKII (H) and pTyr-STAT3 (I) after stimulation with rIL-6 (50ng/ml) in the presence or absence of KN-62 (5μmol/L or 10μmol/L). Data represent means ± SEM from three independent experiments. *P<0.05 vs. control; # P<0.05 vs. IL-6 treatment only.
Similar articles
- Protective Roles of Interferon-γ in Cardiac Hypertrophy Induced by Sustained Pressure Overload.
Kimura A, Ishida Y, Furuta M, Nosaka M, Kuninaka Y, Taruya A, Mukaida N, Kondo T. Kimura A, et al. J Am Heart Assoc. 2018 Mar 19;7(6):e008145. doi: 10.1161/JAHA.117.008145. J Am Heart Assoc. 2018. PMID: 29555642 Free PMC article. - Pressure overload-induced cardiac remodeling and dysfunction in the absence of interleukin 6 in mice.
Lai NC, Gao MH, Tang E, Tang R, Guo T, Dalton ND, Deng A, Tang T. Lai NC, et al. Lab Invest. 2012 Nov;92(11):1518-26. doi: 10.1038/labinvest.2012.97. Epub 2012 Jul 23. Lab Invest. 2012. PMID: 22825686 Free PMC article. - Alleviation of Inflammation and Oxidative Stress in Pressure Overload-Induced Cardiac Remodeling and Heart Failure via IL-6/STAT3 Inhibition by Raloxifene.
Huo S, Shi W, Ma H, Yan D, Luo P, Guo J, Li C, Lin J, Zhang C, Li S, Lv J, Lin L. Huo S, et al. Oxid Med Cell Longev. 2021 Mar 20;2021:6699054. doi: 10.1155/2021/6699054. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33824698 Free PMC article. Retracted. - Stimulation of soluble guanylyl cyclase (sGC) by riociguat attenuates heart failure and pathological cardiac remodelling.
Rüdebusch J, Benkner A, Nath N, Fleuch L, Kaderali L, Grube K, Klingel K, Eckstein G, Meitinger T, Fielitz J, Felix SB. Rüdebusch J, et al. Br J Pharmacol. 2022 Jun;179(11):2430-2442. doi: 10.1111/bph.15333. Epub 2020 Dec 29. Br J Pharmacol. 2022. PMID: 33247945 Review. - Role of CaMKII for signaling and regulation in the heart.
Maier LS. Maier LS. Front Biosci (Landmark Ed). 2009 Jan 1;14(2):486-96. doi: 10.2741/3257. Front Biosci (Landmark Ed). 2009. PMID: 19273080 Review.
Cited by
- Drivers of cardiovascular disease in metabolic dysfunction-associated steatotic liver disease: the threats of oxidative stress.
Minetti ET, Hamburg NM, Matsui R. Minetti ET, et al. Front Cardiovasc Med. 2024 Oct 1;11:1469492. doi: 10.3389/fcvm.2024.1469492. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39411175 Free PMC article. Review. - Unmasking Protein Phosphatase 2A Regulatory Subunit B as a Crucial Factor in the Progression of Dilated Cardiomyopathy.
Lin F, Liang X, Meng Y, Zhu Y, Li C, Zhou X, Hu S, Yi N, Lin Q, He S, Sun Y, Sheng J, Fan H, Li L, Peng L. Lin F, et al. Biomedicines. 2024 Aug 19;12(8):1887. doi: 10.3390/biomedicines12081887. Biomedicines. 2024. PMID: 39200351 Free PMC article. - Cardiac Hypertrophy: From Pathophysiological Mechanisms to Heart Failure Development.
Caturano A, Vetrano E, Galiero R, Salvatore T, Docimo G, Epifani R, Alfano M, Sardu C, Marfella R, Rinaldi L, Sasso FC. Caturano A, et al. Rev Cardiovasc Med. 2022 May 6;23(5):165. doi: 10.31083/j.rcm2305165. eCollection 2022 May. Rev Cardiovasc Med. 2022. PMID: 39077592 Free PMC article. Review. - Dapagliflozin, inflammation and left ventricular remodelling in patients with type 2 diabetes and left ventricular hypertrophy.
Dihoum A, Brown AJ, McCrimmon RJ, Lang CC, Mordi IR. Dihoum A, et al. BMC Cardiovasc Disord. 2024 Jul 12;24(1):356. doi: 10.1186/s12872-024-04022-7. BMC Cardiovasc Disord. 2024. PMID: 38997620 Free PMC article. Clinical Trial. - Identification of immune-related genes and small-molecule drugs in hypertension-induced left ventricular hypertrophy based on machine learning algorithms and molecular docking.
Zhou M, Li T, Lv S, Gan W, Zhang F, Che Y, Yang L, Hou Y, Yan Z, Zeng Z, Zhao W, Yang M. Zhou M, et al. Front Immunol. 2024 Jun 27;15:1351945. doi: 10.3389/fimmu.2024.1351945. eCollection 2024. Front Immunol. 2024. PMID: 38994368 Free PMC article.
References
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. - PubMed
- Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation. 1995;92:1866–1875. - PubMed
- Kaminski KA, Oledzka E, Bialobrzewska K, Kozuch M, Musial WJ, Winnicka MM. The effects of moderate physical exercise on cardiac hypertrophy in interleukin 6 deficient mice. Adv Med Sci. 2007;52:164–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous