HSP70 regulates the function of mitotic centrosomes - PubMed (original) (raw)
HSP70 regulates the function of mitotic centrosomes
Chieh-Ting Fang et al. Cell Mol Life Sci. 2016 Oct.
Abstract
To establish a functional bipolar mitotic spindle, the centrosome expands and matures, acquiring enhanced activities for microtubule (MT) nucleation and assembly at the onset of mitosis. However, the regulatory mechanisms of centrosome maturation and MT assembly from the matured centrosome are largely unknown. In this study, we showed that heat shock protein (HSP) 70 considerably accumulates at the mitotic centrosome during prometaphase to metaphase and is required for bipolar spindle assembly. Inhibition or depletion of HSP70 impaired the function of mitotic centrosome and disrupted MT nucleation and polymerization from the spindle pole, and may thus result in formation of abnormal mitotic spindles. In addition, HSP70 may associate with NEDD1 and γ-tubulin, two pericentriolar material (PCM) components essential for centrosome maturation and MT nucleation. Loss of HSP70 function disrupted the interaction between NEDD1 and γ-tubulin, and reduced their accumulation at the mitotic centrosome. Our results thus demonstrate a role for HSP70 in regulating centrosome integrity during mitosis, and indicate that HSP70 is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle.
Keywords: HSP70; Microtubule nucleation; Microtubule polymerization; Mitotic spindle; Spindle pole.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Fig. 1
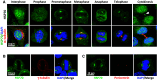
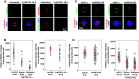
HSP70 accumulates at the mitotic centrosome. a Representative images of cellular distribution of HSP70 in CGL2 cells. Logarithmically growing cells were fixed and stained for HSP70 (green) and α-tubulin (red). The nuclei or chromosomes were counterstained with DAPI (blue). Colocalization of HSP70 with γ-tubulin (b) and pericentrin (c). Logarithmically growing CGL2 cells were fixed and stained for HSP70 (green) and γ-tubulin or pericentrin (red)
Fig. 2
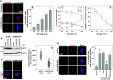
HSP70 is required for assembly of a bipolar mitotic spindle. a, b Inhibition of HSP70 induces spindle defects. CGL2 cells were treated with PES at the indicated concentrations for 24 h and were then fixed and stained for mitotic spindles with antibodies against α-tubulin (red) and γ-tubulin (green). The chromosomes were counterstained with DAPI (blue). The percentage of mitotic cells containing abnormal mitotic spindles was determined using at least 500 mitotic cells from three independent experiments. c PES induces mitotic arrest. Cells were treated as in A and then fixed for analysis of cell cycle distribution. The results (mean ± SD) are from four independent experiments. d PES induces cell death. Cells were treated with PES at the indicated concentrations for 72 h, and then subjected to viability assays. The results (mean ± SD) are from three independent experiments. e Expression level of HSP70 in cells stably depleted of HSP70 (sh-HSPA1A/B). Control (sh-Luc) and sh-HSPA1A/B cells were untreated or treated with arsenic trioxide (ATO) or PES for 14 h; the expression of HSP70 was then examined by immunoblotting. f, g Decreased accumulation of HSP70 at the spindle pole in cells stably depleted of HSP70 (sh-HSPA1A/B). Logarithmically growing control (pLKO.1) and sh-HSPA1A/B cells were fixed and immunostained for HSP70 (green) and α-tubulin (red). Relative HSP70 intensity at the spindle pole was determined (median ± 25 percentiles) from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the control transduced cells (pLKO.1). h, i Depletion of HSP70 induces spindle defects. Control cells (sh-Luc) and cells depleted of HSP70 (sh-HSPA1A/B) were fixed and stained for mitotic spindles as in A. The percentage of mitotic cells containing abnormal mitotic spindles was determined using at least 500 mitotic cells from three independent experiments. Cells stably depleted of HSP70 (sh-HSPA1A/B) were further transduced with a CMV promoter-driven wild type HSP70 (HSP70-wt) or a deletion mutant (HSP70-d5). pFB-Neo is an empty vector and serves as a control. *p < 0.05 by Student’s t test compared to the control depleted cells (pLKO.1). # p < 0.05 by Student’s t test compared to the no ectopic HSP70 control
Fig. 3
Inhibition or depletion of HSP70 induces stabilization of spindle MTs and impedes spindle MT polymerization. Representative images of the mitotic spindle after nocodazole treatment and washout. CGL2 cells were untreated (A) or treated with HSP70 10 μM PES for 16 h (B). At the last 3 h of PES treatment, nocodazole was added into culture medium to 3 μM. Nocodazole was then completely washed out and the cells were incubated in drug-free medium for the indicated time before being immediately fixed and immunostained for α-tubulin (red) and γ-tubulin (green). The chromosomes were counterstained with DAPI (blue). C The number of MT asters in cells. Results are the median ± 25 percentiles of at least 250 mitotic cells for each condition, as determined from three experiments. *p < 0.05 by Mann–Whitney rank sum test as compared with the PES-untreated control. D The fluorescence intensity of centrosomal α-tubulin. Results are the mean ± SD of at least 50 mitotic centrosomes from three independent experiments. *p < 0.05 by Student’s t test as compared with the PES-untreated control. E Representative images of the mitotic spindle after cold treatment. CGL2 cells were untreated (a, b, c) or treated with 10 μM PES (_b_′, _c_′) for 16 h. The cells were then subjected to cold treatment for the indicated time before being stained for mitotic spindles as described in A. For depletion of HSP70 (_b_″, _c_″), the cells were transduced with virion containing the empty vector (pLKO.1) or HSP70-specific shRNAs (sh-HSPA1A/B). After 48 h, the cells were subjected to cold treatment and then immunofluorescence staining. F The percentages of mitotic cells that still exhibited MTs nucleating from the spindle pole after cold treatment for 5 min. G The percentages of mitotic cells with α-tubulin signals remaining at the spindle pole after cold treatment for 30 min. Results are the average of at least 250 cells for each treatment, determined from two experiments. B, bipolar spindle; M, multipolar spindle
Fig. 4
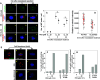
Inhibition or depletion of HSP70 reduces MT nucleation from the spindle pole. a Representative images of cells immunostained for pericentrin (green) and α-tubulin (red). The CGL2 cells were depleted of HSP70 as described in Fig. 2e and then fixed and stained for pericentrin. Scale bar, 10 μm. b Pericentrin volumes at mitotic centrosomes. Pericentrin volume was determined as described in the “Materials and methods”. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the control transduced cells (pLKO.1). c Representative images of PES-treated cells immunostained for α-tubulin (red) and γ-tubulin (green). The cells were treated with 5 μM PES for 16 h and fixed and immunostained as described in Fig. 2a. Scale bar, 10 μm. d, e Relative α-tubulin intensity at the spindle pole in cells lacking HSP70 function. Cells were treated with PES or depleted of HSP70 as described in Fig. 2e. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the untreated or control transduced cells (pLKO.1), as appropriate. f A representative image frame of a time-lapse sequence from a cell stably expressing EGFP-EB1 (HeLa-EB1-GFP). Scale bar 10 μm. g Inhibition or depletion of HSP70 reduces the nucleation of EGFP-EB1 comets. HeLa-EGFP-EB1 cells were treated with PES or depleted of HSP70 as described in Fig. 2e, and then subjected to time-lapse imaging under a confocal microscope as described in the “Materials and methods”. Typically, each comet was visible for three to five frames. Results are the mean ± SD of EB1 comets nucleated from 30 to 50 spindle poles in three independent experiments. *p < 0.05 by Student’s t test compared to the untreated or control transduced cells (pLKO.1), as appropriate
Fig. 5
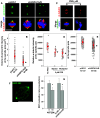
Inhibition or depletion of HSP70 reduces the accumulation of NEDD1 and γ-tubulin at the spindle pole. Representative images of PES-treated (a) or HSP70-depleted (c) CGL2 cells immunostained for NEDD1 (left-side panels) or γ-tubulin (right-side panels). The cells were treated with PES or depleted of HSP70 as described in Fig. 2e, and then fixed and stained for NEDD1 and γ-tubulin. b and d Relative intensities of NEDD1 (left) or γ-tubulin (right) at the spindle pole. Results are the median ± 25 percentiles from three independent experiments. *p < 0.05 by Mann–Whitney rank sum test compared to the untreated or control transduced cells (pLKO.1), as appropriate
Fig. 6
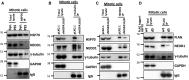
HSP70 associates with NEDD1, and inhibition or depletion of HSP70 disrupts the interaction between NEDD1 and γ-tubulin. a Inhibition of HSP70 by PES treatment reduces the interaction between HSP70 and NEDD1. Mitotic CGL2 cells from untreated cultures were obtained by synchronization with double-thymidine block as described in the “Materials and methods”. The PES-arrested mitotic cells were removed by shaking after treatment with 10 μM PES for 16 h. HSP70 was then immunoprecipitated (IP) using a specific antibody. The immunocomplex was analyzed by immunoblotting using anti-NEDD1 or anti-γ-tubulin. GAPDH and immunoglobin heavy chain (IgG) served as loading controls for total lysate and HSP70 IP, respectively. b and c Depletion of HSP70 impairs the interaction between NEDD1 and γ-tubulin. HSP70 was depleted as described in Fig. 2e. The mitotic cells were then collected as described in a. Gamma-tubulin (b) or NEDD1 (c) in mitotic cells were immunoprecipitated with specific antibodies. The immunocomplex was analyzed by immunoblotting as described in a. d A HSP70 mutant lacking the C-terminal co-chaperone binding domain exhibits a reduced interaction with NEDD1. Mitotic cells from cultures stably expressing the wild type (wt) or the mutant (d5) HSP70 were collected, and ectopic HSP70 was immunoprecipitated with anti-FLAG. The immunocomplex was then analyzed by immunoblotting as described in a
Similar articles
- HSP70 is required for the proper assembly of pericentriolar material and function of mitotic centrosomes.
Fang CT, Kuo HH, Hsu SC, Yih LH. Fang CT, et al. Cell Div. 2019 May 10;14:4. doi: 10.1186/s13008-019-0047-7. eCollection 2019. Cell Div. 2019. PMID: 31110557 Free PMC article. - FAM29A promotes microtubule amplification via recruitment of the NEDD1-gamma-tubulin complex to the mitotic spindle.
Zhu H, Coppinger JA, Jang CY, Yates JR 3rd, Fang G. Zhu H, et al. J Cell Biol. 2008 Dec 1;183(5):835-48. doi: 10.1083/jcb.200807046. Epub 2008 Nov 24. J Cell Biol. 2008. PMID: 19029337 Free PMC article. - Centromere Dysfunction Compromises Mitotic Spindle Pole Integrity.
Gemble S, Simon A, Pennetier C, Dumont M, Hervé S, Meitinger F, Oegema K, Rodriguez R, Almouzni G, Fachinetti D, Basto R. Gemble S, et al. Curr Biol. 2019 Sep 23;29(18):3072-3080.e5. doi: 10.1016/j.cub.2019.07.052. Epub 2019 Sep 5. Curr Biol. 2019. PMID: 31495582 - NEDD1: function in microtubule nucleation, spindle assembly and beyond.
Manning J, Kumar S. Manning J, et al. Int J Biochem Cell Biol. 2007;39(1):7-11. doi: 10.1016/j.biocel.2006.08.012. Epub 2006 Sep 1. Int J Biochem Cell Biol. 2007. PMID: 17005434 Review. - The relative roles of centrosomal and kinetochore-driven microtubules in Drosophila spindle formation.
Gatti M, Bucciarelli E, Lattao R, Pellacani C, Mottier-Pavie V, Giansanti MG, Somma MP, Bonaccorsi S. Gatti M, et al. Exp Cell Res. 2012 Jul 15;318(12):1375-80. doi: 10.1016/j.yexcr.2012.05.001. Epub 2012 May 8. Exp Cell Res. 2012. PMID: 22580224 Review.
Cited by
- Thiostrepton induces spindle abnormalities and enhances Taxol cytotoxicity in MDA-MB-231 cells.
Kuo HH, Yao JS, Yih LH. Kuo HH, et al. Mol Biol Rep. 2024 Aug 21;51(1):927. doi: 10.1007/s11033-024-09863-1. Mol Biol Rep. 2024. PMID: 39168955 Free PMC article. - HSP70 is required for the proper assembly of pericentriolar material and function of mitotic centrosomes.
Fang CT, Kuo HH, Hsu SC, Yih LH. Fang CT, et al. Cell Div. 2019 May 10;14:4. doi: 10.1186/s13008-019-0047-7. eCollection 2019. Cell Div. 2019. PMID: 31110557 Free PMC article. - DnaJB6 is a RanGTP-regulated protein required for microtubule organization during mitosis.
Rosas-Salvans M, Scrofani J, Modol A, Vernos I. Rosas-Salvans M, et al. J Cell Sci. 2019 Jun 3;132(11):jcs227033. doi: 10.1242/jcs.227033. J Cell Sci. 2019. PMID: 31064815 Free PMC article. - Phosphatidylinositol-5-phosphate 4-kinase gamma accumulates at the spindle pole and prevents microtubule depolymerization.
Lin TC, Kuo HH, Wu YC, Pan TS, Yih LH. Lin TC, et al. Cell Div. 2019 Aug 21;14:9. doi: 10.1186/s13008-019-0053-9. eCollection 2019. Cell Div. 2019. PMID: 31452676 Free PMC article. - The Pathophysiological Role of Heat Shock Response in Autoimmunity: A Literature Review.
Androvitsanea A, Stylianou K, Drosataki E, Petrakis I. Androvitsanea A, et al. Cells. 2021 Oct 1;10(10):2626. doi: 10.3390/cells10102626. Cells. 2021. PMID: 34685607 Free PMC article. Review.
References
- Colello D, Mathew S, Ward R, Pumiglia K, LaFlamme SE. Integrins regulate microtubule nucleating activity of centrosome through mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling. J Biol Chem. 2012;287(4):2520–2530. doi: 10.1074/jbc.M111.254128. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials