Cued Reactivation of Motor Learning during Sleep Leads to Overnight Changes in Functional Brain Activity and Connectivity - PubMed (original) (raw)
Cued Reactivation of Motor Learning during Sleep Leads to Overnight Changes in Functional Brain Activity and Connectivity
James N Cousins et al. PLoS Biol. 2016.
Abstract
Sleep plays a role in memory consolidation. This is demonstrated by improved performance and neural plasticity underlying that improvement after sleep. Targeted memory reactivation (TMR) allows the manipulation of sleep-dependent consolidation through intentionally biasing the replay of specific memories in sleep, but the underlying neural basis of these altered memories remains unclear. We use functional magnetic resonance imaging (fMRI) to show a change in the neural representation of a motor memory after targeted reactivation in slow-wave sleep (SWS). Participants learned two serial reaction time task (SRTT) sequences associated with different auditory tones (high or low pitch). During subsequent SWS, one sequence was reactivated by replaying the associated tones. Participants were retested on both sequences the following day during fMRI. As predicted, they showed faster reaction times for the cued sequence after targeted memory reactivation. Furthermore, increased activity in bilateral caudate nucleus and hippocampus for the cued relative to uncued sequence was associated with time in SWS, while increased cerebellar and cortical motor activity was related to time in rapid eye movement (REM) sleep. Functional connectivity between the caudate nucleus and hippocampus was also increased after targeted memory reactivation. These findings suggest that the offline performance gains associated with memory reactivation are supported by altered functional activity in key cognitive and motor networks, and that this consolidation is differentially mediated by both REM sleep and SWS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
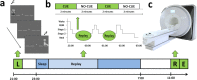
Fig 1. Schematic of experiment design.
(a) Learning (L) of the SRTT task consisted of interleaved blocks of the cued and uncued sequence, and also random blocks. (b) The cued sequence is replayed during periods of SWS in groups of 12 sequences (CUE) and equivalent periods of silence (NO-CUE). (c) Retest (R) of the SRTT takes place the following morning in the MRI scanner, followed shortly afterwards by the explicit memory test outside of the scanner.
Fig 2. Performance improvement at retest.
(a) Comparison of presleep sequence performance to early blocks of sequence retest showed a significant cueing effect. (b) Accuracy improvement was also significantly greater for the cued sequence at early blocks. (c) Performance improvement for both sequences was comparable at late sequence blocks and random blocks that followed. Error bars represent standard error of the mean (SEM) (S1 Data).
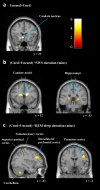
Fig 3. Changes in brain activity after targeted-memory reactivation.
(a) The basic comparison between cued and uncued showed reduced activity in left caudate (−20, 24, −10) for the cued sequence. (b) SWS was associated with enhanced activation in bilateral caudate (16, 8, 20 and −12, 20, 12), and bilateral hippocampi (26, −34, 2 and −22, −34, 6) for the cued sequence relative to the uncued. (b) REM sleep was associated with cueing related activity enhancement in left cerebellum (−32, −54, −44 and 20 −72, −26), left superior parietal cortex (−28, −56, 68 and 22, −54, 38), left sensorimotor cortex (SMC) (−40, −32, 68), left dorsolateral prefrontal cortex (dlPFC) (−30, 34, 28) and right premotor cortex (PMC) (42, −2, 32 and 42, −2, 58). These findings were whole brain corrected (p < 0.05) and displayed as sagittal and coronal projections superimposed on a standard Montreal Neurological Institute (MNI) brain. Colour bar indicates t-values. Anatomical labelling based on peak z-score location.
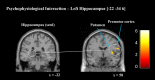
Fig 4. Regions of increased functional connectivity after TMR.
A PPI analysis revealed enhanced connectivity for the cued sequence between left hippocampus (−22, −34, 6) and right putamen (36, −2, 4) and PMC (58, 4, 22). Contrasts displayed as sagittal and coronal projections superimposed on a standard MNI brain. Colour bar indicates t-values. Anatomical labelling based on peak z-score location.
Similar articles
- Neural ensemble reactivation in rapid eye movement and slow-wave sleep coordinate with muscle activity to promote rapid motor skill learning.
Eckert MJ, McNaughton BL, Tatsuno M. Eckert MJ, et al. Philos Trans R Soc Lond B Biol Sci. 2020 May 25;375(1799):20190655. doi: 10.1098/rstb.2019.0655. Epub 2020 Apr 6. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32248776 Free PMC article. - Vocabulary learning benefits from REM after slow-wave sleep.
Batterink LJ, Westerberg CE, Paller KA. Batterink LJ, et al. Neurobiol Learn Mem. 2017 Oct;144:102-113. doi: 10.1016/j.nlm.2017.07.001. Epub 2017 Jul 8. Neurobiol Learn Mem. 2017. PMID: 28697944 Free PMC article. - Targeted memory reactivation of newly learned words during sleep triggers REM-mediated integration of new memories and existing knowledge.
Tamminen J, Lambon Ralph MA, Lewis PA. Tamminen J, et al. Neurobiol Learn Mem. 2017 Jan;137:77-82. doi: 10.1016/j.nlm.2016.11.012. Epub 2016 Nov 15. Neurobiol Learn Mem. 2017. PMID: 27864086 - State of the art on targeted memory reactivation: Sleep your way to enhanced cognition.
Schouten DI, Pereira SI, Tops M, Louzada FM. Schouten DI, et al. Sleep Med Rev. 2017 Apr;32:123-131. doi: 10.1016/j.smrv.2016.04.002. Epub 2016 Apr 21. Sleep Med Rev. 2017. PMID: 27296303 Review. - How Targeted Memory Reactivation Promotes the Selective Strengthening of Memories in Sleep.
Lewis PA, Bendor D. Lewis PA, et al. Curr Biol. 2019 Sep 23;29(18):R906-R912. doi: 10.1016/j.cub.2019.08.019. Curr Biol. 2019. PMID: 31550479 Review.
Cited by
- Promoting memory consolidation during sleep: A meta-analysis of targeted memory reactivation.
Hu X, Cheng LY, Chiu MH, Paller KA. Hu X, et al. Psychol Bull. 2020 Mar;146(3):218-244. doi: 10.1037/bul0000223. Psychol Bull. 2020. PMID: 32027149 Free PMC article. - Mechanisms of Memory Retrieval in Slow-Wave Sleep.
Cairney SA, Sobczak JM, Lindsay S, Gaskell MG. Cairney SA, et al. Sleep. 2017 Sep 1;40(9):zsx114. doi: 10.1093/sleep/zsx114. Sleep. 2017. PMID: 28934526 Free PMC article. - Occipital sleep spindles predict sequence learning in a visuo-motor task.
Lutz ND, Admard M, Genzoni E, Born J, Rauss K. Lutz ND, et al. Sleep. 2021 Aug 13;44(8):zsab056. doi: 10.1093/sleep/zsab056. Sleep. 2021. PMID: 33743012 Free PMC article. - Procedural performance following sleep deprivation remains impaired despite extended practice and an afternoon nap.
Kurniawan IT, Cousins JN, Chong PL, Chee MW. Kurniawan IT, et al. Sci Rep. 2016 Oct 26;6:36001. doi: 10.1038/srep36001. Sci Rep. 2016. PMID: 27782172 Free PMC article. Clinical Trial. - Stimulation Augments Spike Sequence Replay and Memory Consolidation during Slow-Wave Sleep.
Wei Y, Krishnan GP, Marshall L, Martinetz T, Bazhenov M. Wei Y, et al. J Neurosci. 2020 Jan 22;40(4):811-824. doi: 10.1523/JNEUROSCI.1427-19.2019. Epub 2019 Dec 2. J Neurosci. 2020. PMID: 31792151 Free PMC article.
References
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3): 419–57. - PubMed
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15: 161–7. - PubMed
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94(1): 512–8. - PubMed
- Penhune VB, Doyon J. Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage. 2005;26(3): 801–12. - PubMed
Publication types
MeSH terms
Grants and funding
- BB/F003048/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0501110/MRC_/Medical Research Council/United Kingdom
- G1100781/MRC_/Medical Research Council/United Kingdom
- G1000399-2/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials