Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer's disease pathology - PubMed (original) (raw)
Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer's disease pathology
Stephen W Scheff et al. Neurobiol Aging. 2016 Jun.
Abstract
Neuritic amyloid plaques and neurofibrillary tangles are hallmarks of Alzheimer's disease (AD) and are major components used for the clinical diagnosis of this disorder. However, many individuals with no cognitive impairment (NCI) also present at autopsy with high levels of these AD pathologic hallmarks. In this study, we evaluated 15 autopsy cases from NCI individuals with high levels of AD-like pathology (high pathology no cognitive impairment) and compared them to age- and postmortem-matched cohorts of individuals with amnestic mild cognitive impairment and NCI cases with low AD-like pathology (low pathology no cognitive impairment [LPNCI]). Individuals classified as high pathology no cognitive impairment or amnestic mild cognitive impairment had a significant loss of both presynaptic and postsynaptic proteins in the hippocampus compared with those in the LPNCI cohort. In addition, these 2 groups had a significant increase in 3 different markers of oxidative stress compared with that in the LPNCI group. The changes in levels of synaptic proteins are strongly associated with levels of oxidative stress. These data suggest that cognitively older subjects without dementia but with increased levels of AD-like pathology may represent a very early preclinical stage of AD.
Keywords: Aging; Amyloid; Dementia; Hippocampus; Neurodegeneration; Synapses; Temporal lobe.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
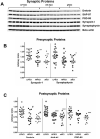
Figure 1
Changes in synaptic protein levels in the hippocampus. Five different synaptic proteins (2 presynaptic and 3 postsynaptic) were analyzed by Western blot with beta actin used as a loading control on the gels (A). Scatter plots showing changes in different synaptic proteins within the hippocampus for each subject from the different cohorts representing different possible stages in the progression of the disease: Low pathology no cognitive impairment (LPNCI), high pathology no cognitive impairment (HPNCI), amnestic mild cognitive impairment (aMCI). (B) Antibodies directed against presynaptic proteins synapsin-1 and synaptophysin are shown. (C) Antibodies directed against postsynaptic proteins drebrin, SAP-97, and PSD-95. Horizontal lines indicate group medians. *p<0.05, **p<0.01, ***p<0.005, #p<0.001 compared to LPNCI.
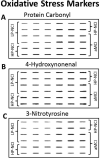
Figure 2
Representative slot-blots for (A) protein carbonyl, (B) 4-hydroxynonenal, and (C) 3-nitrotyrosine fractions from the hippocampus from individuals classified as no cognitive impairment and low AD-like pathology (LP-NCI), no cognitive impairment and high AD-like pathology (HP-NCI), and amnestic mild cognitive impairment (aMCI). The slot-blot shows alterations in staining for the different cohorts for each of the different markers of oxidative stress.
Figure 3
Scatterplots showing changes in the markers of hippocampal oxidative stress among the different cohorts in the present study. The low pathology no cognitive impairment (LPNCI) cohort consistently demonstrated significantly lower levels of oxidative stress compared to the high pathology no cognitive impairment (HPNCI) and amnestic mild cognitive impairment (aMCI) groups for all three of the different markers of oxidative stress. Horizontal lines indicate group medians. *p<0.01, **p<0.001 compared to LPNCI, #p<0.001 compared to HPNCI.
Figure 4
Correlations between different synaptic proteins and markers of oxidative stress in the hippocampus. Almost every comparison showed a negative association between levels of oxidative stress and the levels of various synaptic proteins. One exception was the lack of any observed association between levels of protein carbonyls and levels of the presynaptic marker synaptophysin. Abbreviations: AD, arbitrary units; PSD-95, postsynaptic density-95; SAP-97, synapse associated proteins 97.
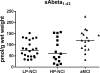
Figure 5
Scatterplots showing the levels of soluble Aβ1-42 in the hippocampus as a function of the different cohorts evaluated. There was no significant difference observed between the two groups with individuals labeled as no cognitive impairment (NCI) (p > 0.1). However, both the low pathology and high pathology NCI groups were significantly lower than the amnestic mild cognitive impairment (aMCI) cohort. Horizontal lines indicate group medians. *p<0.05
Similar articles
- Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice.
Howell MD, Bailey LA, Cozart MA, Gannon BM, Gottschall PE. Howell MD, et al. Acta Neuropathol Commun. 2015 Sep 4;3:54. doi: 10.1186/s40478-015-0233-z. Acta Neuropathol Commun. 2015. PMID: 26337292 Free PMC article. - Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer's disease neuropathology.
Bjorklund NL, Reese LC, Sadagoparamanujam VM, Ghirardi V, Woltjer RL, Taglialatela G. Bjorklund NL, et al. Mol Neurodegener. 2012 May 28;7:23. doi: 10.1186/1750-1326-7-23. Mol Neurodegener. 2012. PMID: 22640423 Free PMC article. - Biomarkers, ketone bodies, and the prevention of Alzheimer's disease.
VanItallie TB. VanItallie TB. Metabolism. 2015 Mar;64(3 Suppl 1):S51-7. doi: 10.1016/j.metabol.2014.10.033. Epub 2014 Oct 30. Metabolism. 2015. PMID: 25468143 Review.
Cited by
- Vesicular Glutamate Transporter Changes in the Cortical Default Mode Network During the Clinical and Pathological Progression of Alzheimer's Disease.
Mi Z, Abrahamson EE, Ryu AY, Malek-Ahmadi M, Kofler JK, Fish KN, Sweet RA, Villemagne VL, Schneider JA, Mufson EJ, Ikonomovic MD. Mi Z, et al. J Alzheimers Dis. 2023;94(1):227-246. doi: 10.3233/JAD-221063. J Alzheimers Dis. 2023. PMID: 37212097 Free PMC article. - Disordered APP metabolism and neurovasculature in trauma and aging: Combined risks for chronic neurodegenerative disorders.
Ikonomovic MD, Mi Z, Abrahamson EE. Ikonomovic MD, et al. Ageing Res Rev. 2017 Mar;34:51-63. doi: 10.1016/j.arr.2016.11.003. Epub 2016 Nov 6. Ageing Res Rev. 2017. PMID: 27829172 Free PMC article. Review. - Loss of precuneus dendritic spines immunopositive for spinophilin is related to cognitive impairment in early Alzheimer's disease.
Mi Z, Abrahamson EE, Ryu AY, Fish KN, Sweet RA, Mufson EJ, Ikonomovic MD. Mi Z, et al. Neurobiol Aging. 2017 Jul;55:159-166. doi: 10.1016/j.neurobiolaging.2017.01.022. Epub 2017 Feb 4. Neurobiol Aging. 2017. PMID: 28259365 Free PMC article. - Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy?
Zedde M, Piazza F, Pascarella R. Zedde M, et al. Biomedicines. 2025 Apr 5;13(4):881. doi: 10.3390/biomedicines13040881. Biomedicines. 2025. PMID: 40299513 Free PMC article. Review. - Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers.
Mesa-Herrera F, Taoro-González L, Valdés-Baizabal C, Diaz M, Marín R. Mesa-Herrera F, et al. Int J Mol Sci. 2019 Aug 4;20(15):3810. doi: 10.3390/ijms20153810. Int J Mol Sci. 2019. PMID: 31382686 Free PMC article. Review.
References
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103(2):373–83. - PubMed
- Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. Journal of neurotrauma. 2008b;25(5):513–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG043375/AG/NIA NIH HHS/United States
- R01 AG042475/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical