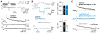Fast, Temperature-Sensitive and Clathrin-Independent Endocytosis at Central Synapses - PubMed (original) (raw)
Fast, Temperature-Sensitive and Clathrin-Independent Endocytosis at Central Synapses
Igor Delvendahl et al. Neuron. 2016.
Abstract
The fusion of neurotransmitter-filled vesicles during synaptic transmission is balanced by endocytotic membrane retrieval. Despite extensive research, the speed and mechanisms of synaptic vesicle endocytosis have remained controversial. Here, we establish low-noise time-resolved membrane capacitance measurements that allow monitoring changes in surface membrane area elicited by single action potentials and stronger stimuli with high-temporal resolution at physiological temperature in individual bona-fide mature central synapses. We show that single action potentials trigger very rapid endocytosis, retrieving presynaptic membrane with a time constant of 470 ms. This fast endocytosis is independent of clathrin but mediated by dynamin and actin. In contrast, stronger stimuli evoke a slower mode of endocytosis that is clathrin, dynamin, and actin dependent. Furthermore, the speed of endocytosis is highly temperature dependent with a Q10 of ∼3.5. These results demonstrate that distinct molecular modes of endocytosis with markedly different kinetics operate at central synapses.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Figure 1. Ultrafast single-AP-evoked endocytosis
(A) Top: Voltage command (Vcommand) used for AP-evoked capacitance recordings in cMFBs. The AP waveform was recorded in a previous experiment with axonal stimulation. An example of a resulting Ca2+ current (ICa) is depicted below. On the right, Vcommand and ICa are shown on an expanded time scale and half-durations are indicated. Middle: Example single-AP-evoked Cm trace (average of 40 consecutive sweeps). Bottom: Corresponding series and membrane resistance (Rs and Rm, respectively). (B) TeNT-LC effectively blocks synaptic vesicle exocytosis in cMFBs. Top: Voltage protocol with 3-ms depolarization from −80 mV to 0 mV. Middle: Pharmacologically isolated Ca2+ current immediately after break-in (control, black) and after 3:00 minutes of whole-cell recording (5 μM TeNT-LC, blue). Bottom: Corresponding Cm traces. Right: Average data of Ca2+ current amplitudes and Cm increase (ΔCm) elicited with 3-ms depolarizations for control (black) and 5 μM TeNT-LC (blue; n represents number of cMFBs). Data are represented as mean ± SEM. (C) Grand average of AP-evoked Cm responses (black, n represents number of cMFBs). Blocking synaptic vesicle exocytosis with 5 μM TeNT-LC (blue) revealed a transient Cm increase not related to exocytosis. Subtraction (gray) shows the time course of endocytosis following a single AP. See also Figure S1.
Figure 2. Highly temperature-sensitive endocytosis at hippocampal and cerebellar mossy fiber synapses
(A) Example traces of Cm recordings in hMFBs evoked by a train of ten stimuli (1-ms depolarizations to +20 mV) delivered at 50 Hz (arrow) at 36° C (red), 30° C (gray), and 24° C (blue). Lower traces represent corresponding membrane and series resistance (Rm and Rs, respectively). (B) Grand average Cm traces recorded at 36° C, 30° C, and 24° C (color code as in A; n represents number of hMFBs). The decay of the grand average traces was best fit with the sum of two exponentials with time constants of 1.1 s (67%) and 11.3 s for 36° C, the sum of two exponentials with time constants of 2.3 s (43%) and 7.4 s for 30° C, and a single exponential function with time constant of 10.7 s for 24° C. (C) Average amplitude-weighted time constant (τw) of the exponential fits to the Cm decay for the three temperatures. Data are represented as mean ± SEM, n is given in B. (D) Left: Histogram of Q10 values by bootstrap analysis of endocytosis rates obtained in hMFBs based on τw data shown in panel C. Right: Corresponding histogram of Q10 values by bootstrap analysis of endocytosis rates obtained in cMFBs using 3-ms depolarizations at 23° C and 36° C (cf. Figure S2A). See also Figure S2.
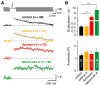
Figure 3. Clathrin-independent single-AP-evoked endocytosis
(A) Top: Voltage command used for AP-evoked Cm recordings in cMFBs. Bottom: Grand averages of AP-evoked Cm responses (n represents number of cMFBs) for control (black), application of a clathrin-inhibitor (pitstop 2, 25 μM; orange), a dynamin-inhibitor (dynasore, 100 μM; red), and an actin-inhibitor (latrunculin A, 25 μM; green). Gray solid lines are exponential fits to the Cm decay. (B) Average time constants of endocytosis and amplitudes of AP-evoked ΔCm with endocytosis inhibitors (color code as in A). The speed of endocytosis was unaltered by pitstop 2 (p = 0.92), but significantly slowed by dynasore and latrunculin A (p < 0.001). Data are represented as mean ± SEM. See also Figure S3.
Figure 4. Distinct molecular modes of endocytosis
(A) Top: Voltage command of 20 APs at a frequency of 300 Hz for Cm recordings in cMFBs. Bottom: Corresponding Ca2+ currents with slight amplitude facilitation. (B) Example Cm traces following 20 APs at 300 Hz for control (black), application of a clathrin-inhibitor (pitstop 2, 25 μM; orange), a dynamin-inhibitor (dynasore, 100 μM; red), an actin-inhibitor (latrunculin A, 25 μM; green), and with TeNT-LC (5 μM, blue). (C) Top: 30-ms voltage step from −80 mV to 0 mV. Bottom: Corresponding Ca2+ current. (D) Example Cm traces following a 30-ms depolarization as shown in C. (E) Summary of the effect of endocytosis inhibitors on exocytosis evoked by different stimuli recorded in cMFBs (color code as in panel B and D). The endocytosis inhibitors dynasore, latrunculin A, and pitstop 2 had no effect on exocytosis evoked by weaker stimuli (1 AP, 20 APs, 1 ms, and 3 ms), whereas TeNT-LC completely blocked synaptic vesicle exocytosis. For 30-ms step pulses, exocytosis was reduced by latrunculin A. Inset: Enlargement of the first three stimulus types on a logarithmic scale. (F) Summary of the effect of endocytosis inhibitors on time constants of endocytosis evoked by different stimuli. The clathrin-inhibitor (pitstop 2, orange) had no effect on fast endocytosis evoked by single APs and AP trains (arrows), but stronger impact on endocytosis evoked by depolarizations of 1, 3, or 30 ms duration. In contrast, a dynamin-inhibitor (dynasore, red) and an actin-inhibitor (latrunculin A, green) reduced the speed of endocytosis evoked by all tested stimuli. Throughout the figure, data are represented as mean ± SEM and asterisks indicate significance level with Kruskal-Wallis tests and post hoc Mann-Whitney-U tests both with Bonferroni-Holm correction. See also Figures S3 and S4.
Comment in
- Catching Up with Ultrafast Endocytosis.
Brockmann MM, Rosenmund C. Brockmann MM, et al. Neuron. 2016 May 4;90(3):423-4. doi: 10.1016/j.neuron.2016.04.027. Neuron. 2016. PMID: 27151632 Review.
Similar articles
- Monitoring clathrin-mediated endocytosis during synaptic activity.
Mueller VJ, Wienisch M, Nehring RB, Klingauf J. Mueller VJ, et al. J Neurosci. 2004 Feb 25;24(8):2004-12. doi: 10.1523/JNEUROSCI.4080-03.2004. J Neurosci. 2004. PMID: 14985443 Free PMC article. - Synaptic Vesicle Endocytosis Occurs on Multiple Timescales and Is Mediated by Formin-Dependent Actin Assembly.
Soykan T, Kaempf N, Sakaba T, Vollweiter D, Goerdeler F, Puchkov D, Kononenko NL, Haucke V. Soykan T, et al. Neuron. 2017 Feb 22;93(4):854-866.e4. doi: 10.1016/j.neuron.2017.02.011. Neuron. 2017. PMID: 28231467 - Clathrin-mediated endocytosis: the physiological mechanism of vesicle retrieval at hippocampal synapses.
Granseth B, Odermatt B, Royle SJ, Lagnado L. Granseth B, et al. J Physiol. 2007 Dec 15;585(Pt 3):681-6. doi: 10.1113/jphysiol.2007.139022. Epub 2007 Jun 28. J Physiol. 2007. PMID: 17599959 Free PMC article. Review. - Dynamin and activity regulate synaptic vesicle recycling in sympathetic neurons.
Lu W, Ma H, Sheng ZH, Mochida S. Lu W, et al. J Biol Chem. 2009 Jan 16;284(3):1930-7. doi: 10.1074/jbc.M803691200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19010787 - Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.
Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM. Augustine GJ, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review.
Cited by
- Membrane transformations of fusion and budding.
Wu LG, Chan CY. Wu LG, et al. Nat Commun. 2024 Jan 2;15(1):21. doi: 10.1038/s41467-023-44539-7. Nat Commun. 2024. PMID: 38167896 Free PMC article. Review. - Functional Electron Microscopy, "Flash and Freeze," of Identified Cortical Synapses in Acute Brain Slices.
Borges-Merjane C, Kim O, Jonas P. Borges-Merjane C, et al. Neuron. 2020 Mar 18;105(6):992-1006.e6. doi: 10.1016/j.neuron.2019.12.022. Epub 2020 Jan 9. Neuron. 2020. PMID: 31928842 Free PMC article. - Spoiled for Choice: Diverse Endocytic Pathways Function at the Cell Surface.
Thottacherry JJ, Sathe M, Prabhakara C, Mayor S. Thottacherry JJ, et al. Annu Rev Cell Dev Biol. 2019 Oct 6;35:55-84. doi: 10.1146/annurev-cellbio-100617-062710. Epub 2019 Jul 5. Annu Rev Cell Dev Biol. 2019. PMID: 31283376 Free PMC article. Review. - Synaptojanin and Endophilin Mediate Neck Formation during Ultrafast Endocytosis.
Watanabe S, Mamer LE, Raychaudhuri S, Luvsanjav D, Eisen J, Trimbuch T, Söhl-Kielczynski B, Fenske P, Milosevic I, Rosenmund C, Jorgensen EM. Watanabe S, et al. Neuron. 2018 Jun 27;98(6):1184-1197.e6. doi: 10.1016/j.neuron.2018.06.005. Neuron. 2018. PMID: 29953872 Free PMC article. - Release Mode Dynamically Regulates the RRP Refilling Mechanism at Individual Hippocampal Synapses.
Kim Y, Lee U, Choi C, Chang S. Kim Y, et al. J Neurosci. 2020 Oct 28;40(44):8426-8437. doi: 10.1523/JNEUROSCI.3029-19.2020. Epub 2020 Sep 28. J Neurosci. 2020. PMID: 32989096 Free PMC article.
References
- Alabi AA, Tsien RW. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. - PubMed
- Cousin MA. Synaptic Vesicle Endocytosis and Endosomal Recycling in Central Nerve Terminals: Discrete Trafficking Routes? Neuroscientist. 2015;21:413–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources