Understanding the Causes and Implications of Endothelial Metabolic Variation in Cardiovascular Disease through Genome-Scale Metabolic Modeling - PubMed (original) (raw)
Review
Understanding the Causes and Implications of Endothelial Metabolic Variation in Cardiovascular Disease through Genome-Scale Metabolic Modeling
Sarah McGarrity et al. Front Cardiovasc Med. 2016.
Abstract
High-throughput biochemical profiling has led to a requirement for advanced data interpretation techniques capable of integrating the analysis of gene, protein, and metabolic profiles to shed light on genotype-phenotype relationships. Herein, we consider the current state of knowledge of endothelial cell (EC) metabolism and its connections to cardiovascular disease (CVD) and explore the use of genome-scale metabolic models (GEMs) for integrating metabolic and genomic data. GEMs combine gene expression and metabolic data acting as frameworks for their analysis and, ultimately, afford mechanistic understanding of how genetic variation impacts metabolism. We demonstrate how GEMs can be used to investigate CVD-related genetic variation, drug resistance mechanisms, and novel metabolic pathways in ECs. The application of GEMs in personalized medicine is also highlighted. Particularly, we focus on the potential of GEMs to identify metabolic biomarkers of endothelial dysfunction and to discover methods of stratifying treatments for CVDs based on individual genetic markers. Recent advances in systems biology methodology, and how these methodologies can be applied to understand EC metabolism in both health and disease, are thus highlighted.
Keywords: endothelium; genetics; metabolic modeling; metabolism; metabolomics; personalized/precision medicine.
Figures
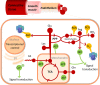
Figure 1
Endothelial metabolism and its links to cellular damage, function, and proliferation control. Metabolism, including glycolysis, pentose phosphate pathway, TCA cycle, fatty acid oxidation, and nitric oxide synthase are represented in red. Useful products of metabolism, NO, ATP, and Ca2+ signaling are shown in green. Damaging side products of metabolism are shown in yellow.
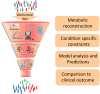
Figure 2
Workflow of GEM construction and contribution to developing new strategies for the clinic. Biochemical data from cell culture and clinical studies are combined to form a comprehensive metabolic reconstruction, which is constrained to form a context-specific GEM and produce biologically well-founded predictions that will suggest future clinical interventions.
References
- WHO. Cardiovascular Diseases (CVDs). World Health Organization; (2016). Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation (2010) 122(Suppl 3):640–57. 10.1161/CIRCULATIONAHA.110.970889 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources