The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes - PubMed (original) (raw)
The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes
Tobias Ritterhoff et al. Nat Commun. 2016.
Abstract
Continuous cycles of nucleocytoplasmic transport require disassembly of transport receptor/Ran-GTP complexes in the cytoplasm. A basic disassembly mechanism in all eukaryotes depends on soluble RanGAP and RanBP1. In vertebrates, a significant fraction of RanGAP1 stably interacts with the nucleoporin RanBP2 at a binding site that is flanked by FG-repeats and Ran-binding domains, and overlaps with RanBP2's SUMO E3 ligase region. Here, we show that the RanBP2/RanGAP1*SUMO1/Ubc9 complex functions as an autonomous disassembly machine with a preference for the export receptor Crm1. We describe three in vitro reconstituted disassembly intermediates, which show binding of a Crm1 export complex via two FG-repeat patches, cargo-release by RanBP2's Ran-binding domains and retention of free Crm1 at RanBP2 after Ran-GTP hydrolysis. Intriguingly, all intermediates are compatible with SUMO E3 ligase activity, suggesting that the RanBP2/RanGAP1*SUMO1/Ubc9 complex may link Crm1- and SUMO-dependent functions.
Figures
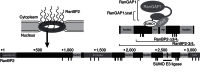
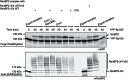
Figure 1. RanBP2 and the RanBP2 complex.
Schematic view of a nuclear pore complex (upper left), of the domain structure of RanBP2 (bottom) and of the RanBP2/RanGAP1*SUMO1/Ubc9 complex (RanBP2 complex, upper right). RanGAP1 is depicted with its N-terminal catalytic domain (large oval), its C-terminal tail domain (Δcat, small oval) and the acidic linker region (arch). Reconstituted versions of the RanBP2 complex used in this work are indicated. CLD, cyclophilin-like domain; IR, internal repeat region of RanBP2 containing its SUMO E3 ligase actvity. Vertical dashes denotes FG-repeats.
Figure 2. The RanBP2-/4 complex specifically interacts with Crm1.
(a) Interaction of transport receptors with the RanBP2-3/4 complex in gel filtration. The RanBP2-3/4 complex (1 μM) was incubated with the indicated transport receptors (2 μM each). Proteins were separated over a Superdex200 5/150 GL gel filtration column. Samples of the input (10%) and of fractions collected at 0.42 CV (column volumes) were analysed by SDS-PAGE and Coomassie staining. *Ovalbumin from buffer; S1, SUMO1. (b) Crm1 co-migrates with the RanBP2-3/4 complex in gel filtration, whereas Importin 13 does not; the elution peaks of Importin 13 and the RanBP2-3/4 complex partially overlap. Gel filtration binding assays were performed as described in a with the RanBP2-3/4 complex, Crm1 (left) and Importin 13 (right). Fractions collected at 0.42–0.50 CV were analysed by SDS-PAGE and Coomassie staining.
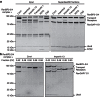
Figure 3. The Crm1/RanBP2 interaction depends on both FG-repeat patches in RanBP2-3/4.
(a) Scheme of RanBP2-3/4 variants in which the two IR-flanking FG-repeat patches were rendered non-functional for transport receptor interaction by F-to-S mutations of each patch (6 mutations for the N-terminal patch=mFG1 and 5 mutations for the C-terminal patch=mFG2 of the E3 ligase region). (b) Crm1 requires both IR-adjacent FG-repeat patches of RanBP2-3/4 for a stable interaction. RanBP2-3/4 complex variants (wild-type and FG-mutants as depicted in a—1 μM each) were incubated with Crm1 (2 μM) and separated over a Superdex200 5/150 GL gel filtration column. Samples were collected at 0.42 CV and analysed by SDS-PAGE and Coomassie staining. S1, SUMO1. (c) Mutations in conserved FG-binding patches of Crm1 reduce binding to the RanBP2-3/4 complex. The RanBP2-3/4 complex (500 nM) was incubated with Crm1 variants (1 μM) and separated over a Superdex200 5/150 GL gel filtration column. Samples were collected at 0.44 CV and analysed by SDS-PAGE and Coomassie staining. (d) Crm1 associates with the endogenous mitotic RanBP2 complex. Immunoprecipitations with the indicated antibodies were performed from nocodazole-arrested HeLa CSH cells. Immunoprecipitates were analysed by αRanBP2 (upper panels), αCrm1 (middle panels) and αRanGAP1 immunoblots (lower panels). (e,f) The FG-repeats flanking the IR-region significantly contribute to the association of Crm1 with the mitotic RanBP2 complex. HEK293T cells were depleted of endogenous RanBP2 by double siRNA treatment and transfected with siRNA resistant, full-length, HA-tagged RanBP2 constructs: wild-type (WT) or a variant lacking the E3 ligase-adjacent FG-repeats (mFG1/2). After nocodazole arrest, cells were lysed, subjected to αHA immunoprecipitation and the amount of co-immunoprecipitated Crm1 was analysed by SDS-PAGE and immunoblotting. As a control, αHA immunoprecipitation was performed from cells that were treated with non-targeting siRNA (NT). Representative αHA (top) and αCrm1* (bottom) immunoblots from αHA immunoprecipitation are shown in e and the quantification of Crm1 signals normalized to the HA-RanBP2 signals from three biological replicates are shown in f. Error bars=s.e.m.; significance was calculated by one-sided paired Student's _t_-test (_P_=0.029); αCrm1*=self-made Crm1 antibody.
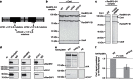
Figure 4. Binding of Crm1 and RanQ69L reduces the structural flexibility of the RanBP2-3/4 complex.
(a) On Crm1 and RanQ69L binding, the Stokes radius of the RanBP2-3/4 complex does not increase. RanBP2-3/4 complex variants (1 μM each), Crm1 (1.5 μM) and RanQ69L (3 μM) were incubated as indicated and separated over Superose6 10/300 GL gel filtration column. Displayed are chromatograms recorded at 280 nm. The molecular weights (calculated in italics and determined by SEC-MALS in bold; Supplementary Fig. 1d) of analysed proteins and protein complexes are indicated. For molecular weight calculations, we assumed a binding stoichiometry between RanQ69L and RanBP2-3/4 of 2:1. Dashed line=elution volume of the RanBP2-3/4 complex. (b) In the presence of RanQ69L, Crm1 binds more strongly to the RanBP2-3/4 complex. Gel filtration binding assays were performed as in a. Samples collected at 0.56 CV were analysed by SDS-PAGE and Coomassie staining. S1, SUMO1. (c) Dissociation constant of the interaction between RanBP2-3/4 and Crm1 determined by MST. Cy3-labelled Crm1 (20 nM) was incubated with RanBP2-3/4 fragments (0.3 nM—10 μM) in the absence and presence of RanQ69L (30 μM) and subjected to MST (laser-power 70%, LED-power 20%). Shown is a summary of three biological replicates per sample with values normalized to the fraction of bound Crm1-Cy3. Error bars=s.e.m. (d) Electron micrographs of uranyl acetate-stained Crm1, RanBP2-3/4 complex and RanBP2-3/4 complex bound to Crm1 and RanQ69L. Images were obtained at 120 keV; representative particles are shown. Crm1 (top row) was purified and imaged directly. The RanBP2-3/4 complex alone (middle row) and bound to Crm1 and RanQ69L (bottom row) were cross-linked with glutaraldehyde, purified and then imaged. Scale bar, 10 nm. (e) Selected 2D class averages of the particles from Crm1, the RanBP2-3/4 complex alone and bound to Crm1 and RanQ69L from d are shown. The average diameter along the longest axis of the analysed particles and the molecular weights are indicated (calculated molecular weights in italics and determined by SEC-MALS in bold; see Supplementary Fig. 1d). Scale bar, 10 nm.
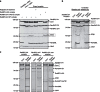
Figure 5. The RanBP2-3/4 complex binds a Crm1 export complex with high affinity via its FG-patches.
(a) The RanBP2-3/4 complex accommodates a Crm1/RanQ69L/SPN1 export complex in an FG-dependent manner. Ratjadone A or mock treated Crm1 (2 μM each), SPN1 (4 μM) and RanQ69L (5 μM) were pre-incubated before adding RanBP2-3/4 complex variants or the RanBP2-Δ3/4 complex (1 μM each) as indicated (export complex=Crm1+SPN1+RanQ69L). The binding reactions were separated over a Superdex200 5/150 GL gel filtration column. Samples collected at 0.42 CV for the RanBP2-3/4 complex and at 0.46 CV for the RanBP2-Δ3/4 complex were analysed by SDS-PAGE and Coomassie staining. Dashed boxes indicate important regions of the gels. (b) The RanBP2-3/4 complex binds to a Crm1/RanQ69L/SPN1 export complex with higher affinity than to Crm1 alone. Binding reactions were set up as in a and applied to a Superdex200 5/150 GL gel filtration column either directly (lanes 1 and 2) or after fivefold dilution (lanes 3 and 4—final concentration of 0.2 μM RanBP2-3/4 complex, 0.4 μM Crm1, 0.8 μM SPN1 and 1 μM RanQ69L). Samples were analysed by SDS-PAGE and Coomassie staining. (c) Increased affinity of the RanBP2-3/4 complex for the Crm1 export complex does not depend on RanBDs and the catalytic domain of RanGAP1. Crm1 (2 μM), SPN1 (4 μM) and RanQ69L (5 μM) were pre-incubated before adding the RanBP2-3/4 complex, the RanBP2-Δ3/4 complex or the RanBP2-3/4 Δcat complex (1 μM each) as indicated. The binding reactions were separated over a Superdex200 5/150 GL gel filtration column. Samples collected at 0.42 CV for the RanBP2-3/4 complex, at 0.46 CV for the RanBP2-Δ3/4 complex and at 0.44 CV for the RanBP2-3/4 Δcat complex were analysed by SDS-PAGE and Coomassie staining. S1, SUMO1.
Figure 6. The RanBP2-3/4 complex can disassemble a Crm1-dependent export complex.
(a) The RanBDs of the RanBP2-3/4 complex dissociate cargo from a Crm1 export complex. Crm1 (2 μM), SPN1 (4 μM) and RanQ69L (1 or 5 μM) were pre-incubated before adding the RanBP2-3/4 complex or the RanBP2-Δ3/4 complex (1 μM each) in the presence or absence of GST-RanBP1 (10 μM) as indicated (export complex=Crm1+SPN1+RanQ69L). Binding reactions were separated over a Superdex200 5/150 GL gel filtration column, and samples collected at 0.42 CV for the RanBP2-3/4 complex and at 0.46 CV for the RanBP2-Δ3/4 complex were analysed by SDS-PAGE and Coomassie staining. S1=SUMO1. Dashed boxes indicate important regions of the gels. (b) The RanBP2-3/4 complex does not dissociate cargo from an export complex formed with a RanBD-binding deficient Ran. Binding assays were performed as in a. The export complex with RanQ69LΔC was formed by incubating Crm1 (10 μM), SPN1 (30 μM) and RanQ69LΔC (50 μM) and purified by gel filtration. For the binding assay, 1 μM purified export complex was used. (c) The RanBDs and RanGAP1*SUMO1 in the RanBP2-3/4 complex can disassemble the Crm1 export complex. Binding assays were performed as in a. RanBP2 complex variants built with RanBP2-3/4 or RanBP2-Δ3/4 and RanGAP1*SUMO1 or RanGAP1Δcat*SUMO1 were added as indicated. Samples were collected at 0.42 CV for the RanBP2-3/4 complex, at 0.44 CV for the RanBP2-3/4 Δcat complex, at 0.46 CV for the RanBP2-Δ3/4 complex and at 0.48 CV for the RanBP2-Δ3/4 Δcat complex. (d) Model of the RanBP2 complex as an autonomous disassembly platform for Crm1 export complexes. From left to right: the partly unfolded RanBP2 complex binds a Crm1 export complex with high affinity with its two IR-adjacent FG-patches thus restricting RanBP2's flexibility. One of the RanBDs of RanBP2 engages the export complex (dashed arrow) resulting in cargo-dissociation and formation of a Crm1/Ran-GTP/RanBD sub-complex. Following its deprotection in the export complex, Ran-GTP is engaged by the catalytic domain of RanGAP1 (dashed arrow). GTP-hydrolysis is induced and Ran-GDP diffuses away leaving behind less stably bound Crm1. The last three stages of this model of Crm1 export complex disassembly were reconstituted in vitro (see text).
Figure 7. Export complex binding is compatible with the E3 ligase activity of the RanBP2-3/4 complex.
Crm1, RanQ69L and SPN1 (2.5 μM each) were pre-incubated (export complex (Crm1+SPN1+RanQ69L)), added to RanBP2-3/4 complex variants (25 nM) as indicated, and the mixture was used as E3 ligase in in vitro SUMOylation reactions containing YFP-Sp100 (500 nM), SUMO E1 enzyme (100 nM), Ubc9 (50 nM) and SUMO1 (10 μM). 10%=the reaction was performed with 2.5 nM RanBP2-3/4 wild-type complex in the absence of export complex components. YFP-Sp100 SUMOylation was analysed by immunoblotting with αGFP (top). Auto-SUMOylation of RanBP2-3/4 was analysed by immunoblotting with αRanBP2 (bottom). RanBP2-3/4*xS1, polySUMOylated RanBP2-3/4.
Similar articles
- Determinants of small ubiquitin-like modifier 1 (SUMO1) protein specificity, E3 ligase, and SUMO-RanGAP1 binding activities of nucleoporin RanBP2.
Gareau JR, Reverter D, Lima CD. Gareau JR, et al. J Biol Chem. 2012 Feb 10;287(7):4740-51. doi: 10.1074/jbc.M111.321141. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194619 Free PMC article. - The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase.
Werner A, Flotho A, Melchior F. Werner A, et al. Mol Cell. 2012 May 11;46(3):287-98. doi: 10.1016/j.molcel.2012.02.017. Epub 2012 Mar 29. Mol Cell. 2012. PMID: 22464730 - Molecular Characterization and Functional Analysis of Annulate Lamellae Pore Complexes in Nuclear Transport in Mammalian Cells.
Raghunayakula S, Subramonian D, Dasso M, Kumar R, Zhang XD. Raghunayakula S, et al. PLoS One. 2015 Dec 7;10(12):e0144508. doi: 10.1371/journal.pone.0144508. eCollection 2015. PLoS One. 2015. PMID: 26642330 Free PMC article. - Mechanistic Insights from Structural Analyses of Ran-GTPase-Driven Nuclear Export of Proteins and RNAs.
Matsuura Y. Matsuura Y. J Mol Biol. 2016 May 22;428(10 Pt A):2025-39. doi: 10.1016/j.jmb.2015.09.025. Epub 2015 Oct 28. J Mol Biol. 2016. PMID: 26519791 Review. - Ran-GTP regulates kinetochore attachment in somatic cells.
Arnaoutov A, Dasso M. Arnaoutov A, et al. Cell Cycle. 2005 Sep;4(9):1161-5. doi: 10.4161/cc.4.9.1979. Epub 2005 Sep 28. Cell Cycle. 2005. PMID: 16082212 Review.
Cited by
- SUMO: From Bench to Bedside.
Chang HM, Yeh ETH. Chang HM, et al. Physiol Rev. 2020 Oct 1;100(4):1599-1619. doi: 10.1152/physrev.00025.2019. Physiol Rev. 2020. PMID: 32666886 Free PMC article. Review. - Aurora B SUMOylation Is Restricted to Centromeres in Early Mitosis and Requires RANBP2.
Di Cesare E, Moroni S, Bartoli J, Damizia M, Giubettini M, Koerner C, Krenn V, Musacchio A, Lavia P. Di Cesare E, et al. Cells. 2023 Jan 19;12(3):372. doi: 10.3390/cells12030372. Cells. 2023. PMID: 36766713 Free PMC article. - Mechanosensitive genomic enhancers potentiate the cellular response to matrix stiffness.
Cosgrove BD, Bounds LR, Taylor CK, Su AL, Rizzo AJ, Barrera A, Crawford GE, Hoffman BD, Gersbach CA. Cosgrove BD, et al. bioRxiv [Preprint]. 2024 Jan 10:2024.01.10.574997. doi: 10.1101/2024.01.10.574997. bioRxiv. 2024. PMID: 38260455 Free PMC article. Preprint. - Differential regulation by CD47 and thrombospondin-1 of extramedullary erythropoiesis in mouse spleen.
Banerjee R, Meyer TJ, Cam MC, Kaur S, Roberts DD. Banerjee R, et al. bioRxiv [Preprint]. 2024 Apr 4:2023.09.28.559992. doi: 10.1101/2023.09.28.559992. bioRxiv. 2024. PMID: 37808833 Free PMC article. Updated. Preprint. - Rhes, a striatal enriched protein, regulates post-translational small-ubiquitin-like-modifier (SUMO) modification of nuclear proteins and alters gene expression.
Rivera O, Sharma M, Dagar S, Shahani N, Ramĺrez-Jarquĺn UN, Crynen G, Karunadharma P, McManus F, Bonneil E, Pierre T, Subramaniam S. Rivera O, et al. Cell Mol Life Sci. 2024 Apr 8;81(1):169. doi: 10.1007/s00018-024-05181-8. Cell Mol Life Sci. 2024. PMID: 38589732 Free PMC article.
References
- Görlich D. & Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660 (1999). - PubMed
- Künzler M. & Hurt E. Targeting of Ran: variation on a common theme? J. Cell. Sci. 114, 3233–3241 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous