Bat Severe Acute Respiratory Syndrome-Like Coronavirus WIV1 Encodes an Extra Accessory Protein, ORFX, Involved in Modulation of the Host Immune Response - PubMed (original) (raw)
Bat Severe Acute Respiratory Syndrome-Like Coronavirus WIV1 Encodes an Extra Accessory Protein, ORFX, Involved in Modulation of the Host Immune Response
Lei-Ping Zeng et al. J Virol. 2016.
Abstract
Bats harbor severe acute respiratory syndrome (SARS)-like coronaviruses (SL-CoVs) from which the causative agent of the 2002-2003 SARS pandemic is thought to have originated. However, despite the fact that a large number of genetically diverse SL-CoV sequences have been detected in bats, only two strains (named WIV1 and WIV16) have been successfully cultured in vitro These two strains differ from SARS-CoV only in containing an extra open reading frame (ORF) (named ORFX), between ORF6 and ORF7, which has no homology to any known protein sequences. In this study, we constructed a full-length cDNA clone of SL-CoV WIV1 (rWIV1), an ORFX deletion mutant (rWIV1-ΔX), and a green fluorescent protein (GFP)-expressing mutant (rWIV1-GFP-ΔX). Northern blotting and fluorescence microscopy indicate that ORFX was expressed during WIV1 infection. A virus infection assay showed that rWIV1-ΔX replicated as efficiently as rWIV1 in Vero E6, Calu-3, and HeLa-hACE2 cells. Further study showed that ORFX could inhibit interferon production and activate NF-κB. Our results demonstrate for the first time that the unique ORFX in the WIV1 strain is a functional gene involving modulation of the host immune response but is not essential for in vitro viral replication.
Importance: Bats harbor genetically diverse SARS-like coronaviruses (SL-CoVs), and some of them have the potential for interspecies transmission. A unique open reading frame (ORFX) was identified in the genomes of two recently isolated bat SL-CoV strains (WIV1 and -16). It will therefore be critical to clarify whether and how this protein contributes to virulence during viral infection. Here we revealed that the unique ORFX is a functional gene that is involved in the modulation of the host immune response but is not essential for in vitro viral replication. Our results provide important information for further exploration of the ORFX function in the future. Moreover, the reverse genetics system we constructed will be helpful for study of the pathogenesis of this group of viruses and to develop therapeutics for future control of emerging SARS-like infections.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
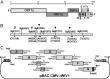
FIG 1
Strategy for construction of an infectious WIV1 BAC clone. (A) Genomic structure of WIV1. (B) The mutations are indicated under the stars. C1575A was used to ablate a natural BglI site at nucleotide 1571 (▽), and T27527C was used to disrupt a potential T7 stop site. The others were for introducing BglI sites (▼). (C) The WIV1 genome was split into eight contiguous cDNAs (A to G): A, nt 1 to 4387; B, nt 4388 to 8032; C1, nt 8033 to 10561; C2, nt 10562 to 12079; D, nt 12080 to 17017; E, nt 17018 to 22468; F, nt 22469 to 27352; G, nt 27353 to 30309. Unique BglI sites were introduced into the fragments by synonymous mutations to make these fragments capable of unidirectional ligation along with native BglI sites in the genome. The original nucleotides are shown above the flanking sequences of corresponding fragments. A poly(A) sequence was added to the 3′ terminus of fragment G. A CMV promoter, HDV ribozyme, and BGH transcriptional terminal signal were inserted into pBeloBAC11 between BamHI and HindIII sites. SacII and AscI sites were introduced between the CMV promoter and ribozyme. Fragments A to G were inserted into the pBAC-CMV plasmid in a single step.
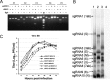
FIG 2
Recovery and characterization of recombinant viruses. (A) Restriction fragment length polymorphism. Amplicons flanking five mutated sites of wild-type and recombinant viruses were digested by BglI. The first amplicon (F1) of wild-type virus can be digested by BglI, and its other four amplicons (F2 to F5) cannot be. In contrast, for amplicons of rWIV1, the first amplicon (F1) cannot be digested by BglI and its other four amplicons (F2 to F5) can be. Lane M, DL2000 DNA ladder (TaKaRa). (B) Detection of viral genomic transcription and replication by Northern blotting. Vero E6 cells were infected with wild-type or recombinant viruses, and intracellular RNA was extracted for Northern blot analysis. Lane 1, wtWIV1; lane 2, rWIV1-ΔX; lane 3, rWIV1; lane 4, uninfected control. (C) Growth kinetics of wild-type and recombinant viruses. Vero E6 cells were infected with wtWIV1 (■), rWIV1 (♢), or rWIV1-ΔX (▲) at an MOI of 1.0 or 0.1 PFU/cell. Cell supernatants were taken at the indicated time points postinfection, and virus titers were determined by plaque assay in Vero E6 cells.
FIG 3
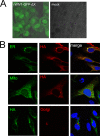
Expression and subcellular location of ORFX protein. (A) The open reading frame of ORFX was replaced by the GFP sequence, and the recombinant virus was rescued. Vero E6 cells were infected with the recombinant virus or mock infected. Green fluorescence was visualized at 24 h postinfection. (B) ORFX protein with an HA tag at the C terminus was expressed in HeLa cells, along with SecG1β-GFP (ER marker), Mito-YFP (mitochondria marker), or B4Gal-Ti-RFP (Golgi marker). The cells were fixed after 24 h and stained with a mouse anti-HA IgG. A Cy3-conjugated goat anti-mouse IgG was used for secondary detection in cells expressing an ER or mitochondrial marker. An FITC-conjugated goat anti-mouse IgG was used for secondary detection in cells expressing a Golgi marker. ORFX protein showed a cytoplasmic distribution and colocalized with the ER maker SecG1β.
FIG 4
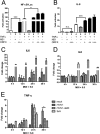
ORFX protein inhibits the production of type I interferon. (A and B) 293T cells seeded in 12-well plates were transfected with 100 ng pIFN-β-Luc, 5 ng pRL-TK, empty vector, an influenza A NS1-expressing plasmid, or increasing doses (100, 200, 400, 600, and 800 ng) of an ORFX-expressing plasmid. Empty vector was added appropriately to ensure that cells in each well were transfected with the same amount of plasmids. The cells were infected with Sendai virus (100 hemagglutinating units/ml) at 24 h posttransfection. Samples were collected at 12 h postinfection, followed by dual-luciferase assay. The results were expressed as the firefly luciferase value normalized to that of Renilla luciferase. The relative expression of IFN-β mRNA was determined by quantitative RT-PCR and normalized to the expression level of GAPDH mRNA. (C) The expression of the NS1 and ORFX proteins was analyzed by Western blotting with an antibody against HA tag. The experiments were replicated three times. (D and E) For the IRF3 translocation assay, 293T cells were transfected with empty vector-, NS1-, or ORFX-expressing plasmid. After 24 h, the cells were infected with Sendai virus to induce IRF3 nuclear translocation. The cells were fixed at 8 h postinfection and stained with anti-HA IgG. A goat anti-Sendai virus polyclonal IgG was used to stain the cells transfected with empty vector. A rabbit anti-IRF3 polyclonal IgG was used to label IRF3. The white arrow indicates IRF3 nuclear translocation. The relative IRF3 translocation ratios were calculated for each group by counting the number of IRF3 nuclear translocation cells (randomly selected from at least 4 fields) and dividing by the total number of infected or transfected cells. The IRF3 nuclear translocation efficiency of each group was expressed as the percentage of their relative IRF3 translocation ratios to that of the control (cells transfected with empty vector). (F) Calu-3 cells were mock infected or infected with rWIV1 or rWIV1-ΔX (MOI of 5) or SeV (100 HAU/ml). At 4, 12, 24, and 30 h postinfection, the cell RNA was extracted and used for quantitative RT-PCR of the expression level of IFN-β mRNA. The experiment was performed in triplicate and replicated twice. (G) Vero E6 cells were pretreated with indicated amount of IFN-β, infected with wtWIV1, rWIV1, or rWIV1-ΔX at an MOI of 0.1 PFU/cell, and posttreated with IFN-β. Viral replication was analyzed at 24 h postinfection by plaque assay. The experiment was performed in triplicate and replicated twice. The differences between selected groups were significant, with P values of less than 0.05, as follows: 0.0049 (*; bars 4 and 6 in panel A), 0.0008 (**; bars 6 and 7 in panel A), 0.0072 (*; bars 4 and 6 in panel B), 0.018 (*; bars for rWIV1 and rWIV1-ΔX in panel F), and <0.0001 (*** in panel G).
FIG 5
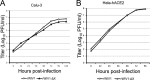
Comparison of viral replication efficiencies of rWIV1-ΔX and rWIV1 in IFN-competent cells. Calu-3 (A) and HeLa-hACE2 (B) cells were infected with rWIV1 or rWIV1-ΔX at an MOI of 0.001. Samples were collected at 0, 12, 24, 36, 48, 72, 96, and 120 h postinfection. The viral titers were measured by plaque assay.
FIG 6
ORFX protein activates NF-κB. 293T cells were transfected with 100 ng pNF-κB-Luc, 10 ng pRL-TK, empty vector, an NS1-expressing plasmid, or increasing amounts (200, 400, and 600 ng) of an ORFX-expressing plasmid. After 24 h, the cells were treated with TNF-α. (A) Dual-luciferase activity was determined after 6 h. The results were expressed as the firefly luciferase activity normalized to that of Renilla luciferase. (B) The relative expression of IL-8 mRNA was quantified through quantitative RT-PCR and normalized to that of GAPDH mRNA. Differences between selected groups were significant, with P value less than 0.05, as follows: <0.0001 (***; bars 1 and 3 in panel A), 0.0339 (*; bars 4 and 7 in panel A), and 0.0002 (***; bars 4 and 6 in panel B). n.s., not significant. The experiments were performed three times. (C to E) The RNA extracted from Calu-3 cells for Fig. 4 was used for quantification of the expression of IL-6 (C), IL-8 (D), and TNF-α (E) mRNAs.
Similar articles
- Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor.
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Ge XY, et al. Nature. 2013 Nov 28;503(7477):535-8. doi: 10.1038/nature12711. Epub 2013 Oct 30. Nature. 2013. PMID: 24172901 Free PMC article. - Bat SARS-Like WIV1 coronavirus uses the ACE2 of multiple animal species as receptor and evades IFITM3 restriction via TMPRSS2 activation of membrane fusion.
Zheng M, Zhao X, Zheng S, Chen D, Du P, Li X, Jiang D, Guo JT, Zeng H, Lin H. Zheng M, et al. Emerg Microbes Infect. 2020 Dec;9(1):1567-1579. doi: 10.1080/22221751.2020.1787797. Emerg Microbes Infect. 2020. PMID: 32602823 Free PMC article. - SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.
Sims AC, Burkett SE, Yount B, Pickles RJ. Sims AC, et al. Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review. - Inactivated SARS-CoV-2 Vaccine Shows Cross-Protection against Bat SARS-Related Coronaviruses in Human ACE2 Transgenic Mice.
Liu MQ, Jiang RD, Guo J, Chen Y, Yang DS, Wang X, Lin HF, Li A, Li B, Hu B, Wang ZJ, Yang XL, Shi ZL. Liu MQ, et al. J Virol. 2022 Apr 27;96(8):e0016922. doi: 10.1128/jvi.00169-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343762 Free PMC article. - Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.
Tan YJ, Lim SG, Hong W. Tan YJ, et al. Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review.
Cited by
- Angiotensin-converting enzyme 2: The old door for new severe acute respiratory syndrome coronavirus 2 infection.
Tan HW, Xu YM, Lau ATY. Tan HW, et al. Rev Med Virol. 2020 Sep;30(5):e2122. doi: 10.1002/rmv.2122. Epub 2020 Jun 30. Rev Med Virol. 2020. PMID: 32602627 Free PMC article. Review. - Interplay between co-divergence and cross-species transmission in the evolutionary history of bat coronaviruses.
Leopardi S, Holmes EC, Gastaldelli M, Tassoni L, Priori P, Scaravelli D, Zamperin G, De Benedictis P. Leopardi S, et al. Infect Genet Evol. 2018 Mar;58:279-289. doi: 10.1016/j.meegid.2018.01.012. Epub 2018 Jan 30. Infect Genet Evol. 2018. PMID: 29355607 Free PMC article. - Cross-neutralization of SARS coronavirus-specific antibodies against bat SARS-like coronaviruses.
Zeng LP, Ge XY, Peng C, Tai W, Jiang S, Du L, Shi ZL. Zeng LP, et al. Sci China Life Sci. 2017 Dec;60(12):1399-1402. doi: 10.1007/s11427-017-9189-3. Epub 2017 Nov 10. Sci China Life Sci. 2017. PMID: 29134417 Free PMC article. No abstract available. - The broad-spectrum antiviral recommendations for drug discovery against COVID-19.
Hazafa A, Ur-Rahman K, Haq IU, Jahan N, Mumtaz M, Farman M, Naeem H, Abbas F, Naeem M, Sadiqa S, Bano S. Hazafa A, et al. Drug Metab Rev. 2020 Aug;52(3):408-424. doi: 10.1080/03602532.2020.1770782. Epub 2020 Jun 17. Drug Metab Rev. 2020. PMID: 32546018 Free PMC article. Review.
References
- Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. doi:10.1016/S0140-6736(03)13077-2. - DOI - PMC - PubMed
- Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538. doi:10.1038/nature12711. - DOI - PMC - PubMed
- Yuan J, Hon CC, Li Y, Wang D, Xu G, Zhang H, Zhou P, Poon LL, Lam TT, Leung FC, Shi Z. 2010. Intraspecies diversity of SARS-like coronaviruses in Rhinolophus sinicus and its implications for the origin of SARS coronaviruses in humans. J Gen Virol 91:1058–1062. doi:10.1099/vir.0.016378-0. - DOI - PubMed
- Drexler JF, Gloza-Rausch F, Glende J, Corman VM, Muth D, Goettsche M, Seebens A, Niedrig M, Pfefferle S, Yordanov S, Zhelyazkov L, Hermanns U, Vallo P, Lukashev A, Muller MA, Deng H, Herrler G, Drosten C. 2010. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol 84:11336–11349. doi:10.1128/JVI.00650-10. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous