Viral entry pathways: the example of common cold viruses - PubMed (original) (raw)
Review
Viral entry pathways: the example of common cold viruses
Dieter Blaas. Wien Med Wochenschr. 2016 May.
Abstract
For infection, viruses deliver their genomes into the host cell. These nucleic acids are usually tightly packed within the viral capsid, which, in turn, is often further enveloped within a lipid membrane. Both protect them against the hostile environment. Proteins and/or lipids on the viral particle promote attachment to the cell surface and internalization. They are likewise often involved in release of the genome inside the cell for its use as a blueprint for production of new viruses. In the following, I shall cursorily discuss the early more general steps of viral infection that include receptor recognition, uptake into the cell, and uncoating of the viral genome. The later sections will concentrate on human rhinoviruses, the main cause of the common cold, with respect to the above processes. Much of what is known on the underlying mechanisms has been worked out by Renate Fuchs at the Medical University of Vienna.
Bei der Infizierung schleusen Viren ihr Genom in die Wirtszelle ein. Deren Nukleinsäuren befinden sich gewöhnlich gut verpackt innerhalb des Viruskapsids, welches wiederum häufig zusätzlich von einer Lipidmembran umhüllt ist. Beides schützt sie vor einer feindlichen Umgebung. Proteine und/oder Lipide auf dem Viruspartikel unterstützen die Anlagerung an die Zelloberfläche und die Internalisierung. Ebenso sind sie oftmals an der Freisetzung des Genoms in der Zelle beteiligt, welches als Vorlage für die Produktion neuer Viren dient. Im Folgenden werden die frühen, eher allgemeinen Schritte der Virusinfektion kursorisch dargestellt, dazu gehören Rezeptorerkennung, Aufnahme in die Zelle und Freisetzung des Virusgenoms. Im Hinblick auf die genannten Abläufe liegt der Schwerpunkt in den weiteren Abschnitten auf humanen Rhinoviren als Hauptursache für den Schnupfen. Viele der bisherigen Erkenntnisse in Bezug auf die zugrunde liegenden Mechanismen sind von Renate Fuchs, Medizinische Universität Wien, erarbeitet worden.
Keywords: Endocytosis; Genome release; Lysosome; Rhinovirus; Uncoating.
Figures
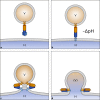
Fig. 1
Fusion of enveloped viruses with a cellular membrane. a A viral envelope protein (often a trimer, as depicted) harbors a fusion peptide that is poorly solvent accessible. b On exposure to the acidic pH inside endosomes, structural changes occur that result in exposure of the fusion peptide and its insertion into the endosomal membrane. c Conformational rearrangements of multiple envelope proteins (just one trimer is shown) force the membranes into close apposition resulting in hemifusion without a fusion pore and only partial mixing of the lipids in one of the leaflets. d Complete fusion resulting in the nucleoprotein/nucleic acid (brown) accessing the cytosol. V virus, H host, (V) designates the residual viral membrane patch that remains after the contents have been transferred into the cytosol. Note that it finally becomes completely integrated into the host membrane with mixing of the lipids (not shown)
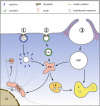
Fig. 2
Simplified view of the major entry pathways. 1 Clathrin-dependent endocytosis. Clathrin-coated (CC) pits are formed at the plasma membrane and mature into clathrin-coated vesicles. These are severed from the plasma membrane by dynamin that forms rings around the necks. Once inside the cytosol, the coat is removed by uncoating ATPases, making the membrane accessible for fusion with other vesicles. Maturation and fusion results in the formation of early endosomes (EE); these mature further into late endosomes (LE) and/or fuse with LE. During the process, the pH continuously decreases from neutral to about 5.6, depending on the cell type. LE finally fuse with lysosomes (L), where the luminal content is degraded by hydrolases whose activity is maximal around pH 5. A side step from EE leads to the perinuclear recycling compartment (PNRE). Some ligands (e. g., transferrin) are returned to the plasma membrane via recycling endosomes (RE). 2 Caveolae (Cav) feature a particular lipid composition rich in cholesterol and glycosyl phosphoinositol-linked proteins, and a more translucent coat of cavin. They can be shuttled to endosomes but also to the Golgi (not shown here). 3 Macropinosomes (MP) form under the direction of actin fibers and transport extracellular liquid but also membrane-bound ligands. They travel to lysosomes for fusion and degradation of their content, but there is a connection to EE as well. Vesicles are often ferried along actin fibers and microtubules via motor proteins like kinesin and dynein (as indicated for RE)
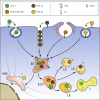
Fig. 3
Simplified view of the entry of major receptor-group rhinoviruses binding ICAM-1 (RV-B14, RV-A89), the minor receptor-group rhinovirus binding LDLR (RV-A2), and the heparan sulfate-binding variant of the major-group virus RV-A8v. On attaching to their respective receptors, RV-A2 and RV-A89 are taken up into coated and RV-B14 and RV-A8v into non-coated vesicles and tubules. RV-A2 is shuttled to early endosomes where the pH is about 5.8 in HeLa cells. On further acidification to a pH below 5.7, RV-A2 releases the RNA through a pore and the remaining capsid proteins are transferred to lysosomes for degradation. RV-A8v enters by a pathway not involving clathrin in macropinocytic vesicles but also tubules (not shown); whether it releases its RNA through holes in the membrane or via lysis of the endosomes is not known. RV-B14 lyses the endosomal membrane and RNA, as well as viral protein, arrives in the cytosol. In this case, no lysosomal degradation is observed. RV-A89 travels to recycling endosomes for uncoating, whether it disrupts the endosomes is not known. LDLR low-density lipoprotein receptor, ICAM-1 intercellular adhesion molecule 1, EL early endosome, LL late endosome, PNRC perinuclear recycling compartment
Similar articles
- Rhinoviruses and cells: molecular aspects.
Rowlands DJ. Rowlands DJ. Am J Respir Crit Care Med. 1995 Oct;152(4 Pt 2):S31-5. doi: 10.1164/ajrccm/152.4_Pt_2.S31. Am J Respir Crit Care Med. 1995. PMID: 7551409 Review. - ICAM-1 induced rearrangements of capsid and genome prime rhinovirus 14 for activation and uncoating.
Hrebík D, Füzik T, Gondová M, Šmerdová L, Adamopoulos A, Šedo O, Zdráhal Z, Plevka P. Hrebík D, et al. Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2024251118. doi: 10.1073/pnas.2024251118. Proc Natl Acad Sci U S A. 2021. PMID: 33947819 Free PMC article. - Chemical Evolution of Rhinovirus Identifies Capsid-Destabilizing Mutations Driving Low-pH-Independent Genome Uncoating.
Murer L, Petkidis A, Vallet T, Vignuzzi M, Greber UF. Murer L, et al. J Virol. 2022 Jan 26;96(2):e0106021. doi: 10.1128/JVI.01060-21. Epub 2021 Oct 27. J Virol. 2022. PMID: 34705560 Free PMC article. - Viral uncoating is directional: exit of the genomic RNA in a common cold virus starts with the poly-(A) tail at the 3'-end.
Harutyunyan S, Kumar M, Sedivy A, Subirats X, Kowalski H, Köhler G, Blaas D. Harutyunyan S, et al. PLoS Pathog. 2013;9(4):e1003270. doi: 10.1371/journal.ppat.1003270. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592991 Free PMC article. - Uncoating of human rhinoviruses.
Fuchs R, Blaas D. Fuchs R, et al. Rev Med Virol. 2010 Sep;20(5):281-97. doi: 10.1002/rmv.654. Rev Med Virol. 2010. PMID: 20629045 Review.
Cited by
- Loss of Olfactory Function-Early Indicator for Covid-19, Other Viral Infections and Neurodegenerative Disorders.
Rebholz H, Braun RJ, Ladage D, Knoll W, Kleber C, Hassel AW. Rebholz H, et al. Front Neurol. 2020 Oct 26;11:569333. doi: 10.3389/fneur.2020.569333. eCollection 2020. Front Neurol. 2020. PMID: 33193009 Free PMC article. Review. - Rhinovirus replication and innate immunity in highly differentiated human airway epithelial cells.
Warner SM, Wiehler S, Michi AN, Proud D. Warner SM, et al. Respir Res. 2019 Jul 12;20(1):150. doi: 10.1186/s12931-019-1120-0. Respir Res. 2019. PMID: 31299975 Free PMC article. - Interplay between hypoxia and inflammation contributes to the progression and severity of respiratory viral diseases.
Bhattacharya S, Agarwal S, Shrimali NM, Guchhait P. Bhattacharya S, et al. Mol Aspects Med. 2021 Oct;81:101000. doi: 10.1016/j.mam.2021.101000. Epub 2021 Jul 19. Mol Aspects Med. 2021. PMID: 34294412 Free PMC article. Review. - Capsid opening enables genome release of iflaviruses.
Škubník K, Sukeník L, Buchta D, Füzik T, Procházková M, Moravcová J, Šmerdová L, Přidal A, Vácha R, Plevka P. Škubník K, et al. Sci Adv. 2021 Jan 1;7(1):eabd7130. doi: 10.1126/sciadv.abd7130. Print 2021 Jan. Sci Adv. 2021. PMID: 33523856 Free PMC article. - "Anosmia" the mysterious collateral damage of COVID-19.
Ahmed AK, Sayad R, Mahmoud IA, El-Monem AMA, Badry SH, Ibrahim IH, Hafez MH, El-Mokhtar MA, Sayed IM. Ahmed AK, et al. J Neurovirol. 2022 Apr;28(2):189-200. doi: 10.1007/s13365-022-01060-9. Epub 2022 Mar 5. J Neurovirol. 2022. PMID: 35249186 Free PMC article. Review.
References
- Vasiljevic S, Beale EV, Bonomelli C, Easthope IS, Pritchard LK, Seabright GE, et al. Redirecting adenoviruses to tumour cells using therapeutic antibodies: Generation of a versatile human bispecific adaptor. Mol Immunol. 2015;68(2A):1413–1429. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical