Radical SAM catalysis via an organometallic intermediate with an Fe-[5'-C]-deoxyadenosyl bond - PubMed (original) (raw)
Radical SAM catalysis via an organometallic intermediate with an Fe-[5'-C]-deoxyadenosyl bond
Masaki Horitani et al. Science. 2016.
Abstract
Radical S-adenosylmethionine (SAM) enzymes use a [4Fe-4S] cluster to cleave SAM to initiate diverse radical reactions. These reactions are thought to involve the 5'-deoxyadenosyl radical intermediate, which has not yet been detected. We used rapid freeze-quenching to trap a catalytically competent intermediate in the reaction catalyzed by the radical SAM enzyme pyruvate formate-lyase activating enzyme. Characterization of the intermediate by electron paramagnetic resonance and (13)C, (57)Fe electron nuclear double-resonance spectroscopies reveals that it contains an organometallic center in which the 5' carbon of a SAM-derived deoxyadenosyl moiety forms a bond with the unique iron site of the [4Fe-4S] cluster. Discovery of this intermediate extends the list of enzymatic bioorganometallic centers to the radical SAM enzymes, the largest enzyme superfamily known, and reveals intriguing parallels to B12 radical enzymes.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Fig. 1
Activation of PFL by PFL-AE, with concomitant cleavage of SAM to methionine and 5′-deoxyadenosine.
Fig. 2
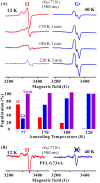
EPR spectra showing the formation of the PFL glycyl radical (G•) from Ω. (A, upper) EPR spectra of mixture of photoreduced PFL-AE and PFL/SAM freeze-quenched at ∼77K (500 ms) and stored at 77 K, and then annealed at progressively higher T for indicated times (See SI). At 12 K, the spectrum of G• radical is highly saturated and its amplitude diminished; at 40 K, signal from rapidly relaxing Ω is correspondingly diminished. The spectra here have had the residual intensities at both temperatures subtracted out (See SI, Fig. S2), with one exception. Ω is completely lost after annealing at 220 K; the dashed curve shows the residual signal from saturated G• radical. (A, lower) Populations of Ω and G• radical relative to the final (220 K) G• radical concentration taken as 100%, as derived from EPR spectra (See SI). (B) X-band EPR spectra for photoreduced PFL-AE freeze-quenched (77K) 500 ms after mixing with PFL G734A/SAM, with spectra collected at 12 and 40 K. Conditions: microwave frequency = 9.23 GHz, microwave power = 1 mW, 100 kHz modulation amplitude = 8 G, T, as indicated; the gain at a given T is fixed.
Fig. 3
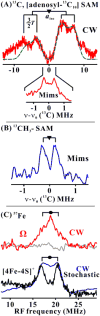
35 GHz ENDOR spectra at _g_⊥ for photoreduced PFL-AE freeze-quenched with PFL/SAM (See SI for details). To first order, an ENDOR spectrum of an I = 1/2 nucleus (N) in a frozen solution comprises a superposition of signals from different orientations, each signal a doublet at frequencies, ν± = |ν(N) ± A/2|, where ν(N) is the nuclear larmor frequency and A is the orientation-dependent hyperfine coupling.(23) For 13C, A/2 ≪ ν(13C) and it is convenient to plot spectra vs ν - ν(13C). For 57Fe, ν(57Fe) ≪ A/2 and spectra are plotted vs ν.(A) 13C CW ENDOR for [adenosyl-13C10] SAM. Best match simulation to axial hyperfine tensor (See SI), green dash line. Simulation parameters, a_iso = 9.4 MHz, 2_T = 5.3 MHz and β = 90°. Conditions: microwave frequency = 35.39 GHz, microwave power = 1 mW, 100 kHz modulation amplitude = 1.3 G, rf sweep rate = 1 MHz/s and T = 2 K. Inset: Mims ENDOR spectrum. Conditions: microwave frequency = 35.20 GHz, MW pulse length, (π/2) = 50 ns, τ = 500 ns and T = 2 K. (B) Mims ENDOR spectrum from [methyl-13C] SAM. Conditions: microwave frequency = 35.08 GHz, MW pulse length, (π/2) = 50 ns, τ = 500 ns and T = 2 K. (C) 57Fe CW ENDOR for 57Fe enriched Ω (prepared using [adenosyl-13C10] SAM) and photoreduced PFL-AE. Upper: CW ENDOR spectra for 57Fe-enriched (red) and natural abundance (gray) rfq samples. Lower: Frequency sweep and randomly hopped stochastic CW ENDOR spectra (23) for 57Fe-enriched reduced PFL-AE. Conditions: microwave frequency = 35.45 GHz and 35.07GHz for rfq and 57Fe-enriched reduced PFL-AE, respectively, microwave power = 1 mW, 100 kHz modulation amplitude = 1.3 G, rf sweep rate = 1 MHz/s, stochastic CW ENDOR cycle; rf-on = 3 ms, rf-off = 1 ms, sample collection time = 3 ms, and T = 2 K.
Fig. 4
Model for bio-organometallic intermediate, Ω. Whether methionine remains coordinated to the unique iron is not currently known.
Similar articles
- Paradigm Shift for Radical S-Adenosyl-l-methionine Reactions: The Organometallic Intermediate Ω Is Central to Catalysis.
Byer AS, Yang H, McDaniel EC, Kathiresan V, Impano S, Pagnier A, Watts H, Denler C, Vagstad AL, Piel J, Duschene KS, Shepard EM, Shields TP, Scott LG, Lilla EA, Yokoyama K, Broderick WE, Hoffman BM, Broderick JB. Byer AS, et al. J Am Chem Soc. 2018 Jul 18;140(28):8634-8638. doi: 10.1021/jacs.8b04061. Epub 2018 Jul 6. J Am Chem Soc. 2018. PMID: 29954180 Free PMC article. - Mechanism of Radical Initiation in the Radical S-Adenosyl-l-methionine Superfamily.
Broderick WE, Hoffman BM, Broderick JB. Broderick WE, et al. Acc Chem Res. 2018 Nov 20;51(11):2611-2619. doi: 10.1021/acs.accounts.8b00356. Epub 2018 Oct 15. Acc Chem Res. 2018. PMID: 30346729 Free PMC article. Review. - Electron-nuclear double resonance spectroscopic evidence that S-adenosylmethionine binds in contact with the catalytically active 4Fe-4S cluster of pyruvate formate-lyase activating enzyme.
Walsby CJ, Hong W, Broderick WE, Cheek J, Ortillo D, Broderick JB, Hoffman BM. Walsby CJ, et al. J Am Chem Soc. 2002 Mar 27;124(12):3143-51. doi: 10.1021/ja012034s. J Am Chem Soc. 2002. PMID: 11902903 - Spectroscopic approaches to elucidating novel iron-sulfur chemistry in the "radical-Sam" protein superfamily.
Walsby CJ, Ortillo D, Yang J, Nnyepi MR, Broderick WE, Hoffman BM, Broderick JB. Walsby CJ, et al. Inorg Chem. 2005 Feb 21;44(4):727-41. doi: 10.1021/ic0484811. Inorg Chem. 2005. PMID: 15859242 - Radical SAM enzymes: Nature's choice for radical reactions.
Broderick JB, Broderick WE, Hoffman BM. Broderick JB, et al. FEBS Lett. 2023 Jan;597(1):92-101. doi: 10.1002/1873-3468.14519. Epub 2022 Oct 27. FEBS Lett. 2023. PMID: 36251330 Free PMC article. Review.
Cited by
- Chemical Antiquity in Metabolism.
Mrnjavac N, Schwander L, Brabender M, Martin WF. Mrnjavac N, et al. Acc Chem Res. 2024 Aug 20;57(16):2267-2278. doi: 10.1021/acs.accounts.4c00226. Epub 2024 Jul 31. Acc Chem Res. 2024. PMID: 39083571 Free PMC article. - Biosynthesis of the sactipeptide Ruminococcin C by the human microbiome: Mechanistic insights into thioether bond formation by radical SAM enzymes.
Balty C, Guillot A, Fradale L, Brewee C, Lefranc B, Herrero C, Sandström C, Leprince J, Berteau O, Benjdia A. Balty C, et al. J Biol Chem. 2020 Dec 4;295(49):16665-16677. doi: 10.1074/jbc.RA120.015371. Epub 2020 Sep 24. J Biol Chem. 2020. PMID: 32972973 Free PMC article. - The Atypical Cobalamin-Dependent _S_-Adenosyl-l-Methionine Nonradical Methylase TsrM and Its Radical Counterparts.
Ulrich EC, Drennan CL. Ulrich EC, et al. J Am Chem Soc. 2022 Apr 6;144(13):5673-5684. doi: 10.1021/jacs.1c12064. Epub 2022 Mar 28. J Am Chem Soc. 2022. PMID: 35344653 Free PMC article. - Paradigm Shift for Radical S-Adenosyl-l-methionine Reactions: The Organometallic Intermediate Ω Is Central to Catalysis.
Byer AS, Yang H, McDaniel EC, Kathiresan V, Impano S, Pagnier A, Watts H, Denler C, Vagstad AL, Piel J, Duschene KS, Shepard EM, Shields TP, Scott LG, Lilla EA, Yokoyama K, Broderick WE, Hoffman BM, Broderick JB. Byer AS, et al. J Am Chem Soc. 2018 Jul 18;140(28):8634-8638. doi: 10.1021/jacs.8b04061. Epub 2018 Jul 6. J Am Chem Soc. 2018. PMID: 29954180 Free PMC article. - _S_-Adenosyl-l-ethionine is a Catalytically Competent Analog of _S_-Adenosyl-l-methione (SAM) in the Radical SAM Enzyme HydG.
Impano S, Yang H, Shepard EM, Swimley R, Pagnier A, Broderick WE, Hoffman BM, Broderick JB. Impano S, et al. Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4666-4672. doi: 10.1002/anie.202014337. Epub 2020 Dec 1. Angew Chem Int Ed Engl. 2021. PMID: 33935588 Free PMC article.
References
- Sicoli G, et al. Fine-tuning of a radical-based reaction by radical S-adenosyl-L-methionine tryptophan lyase. Science. 2016;351:1320. - PubMed
- Walsby CJ, Ortillo D, Broderick WE, Broderick JB, Hoffman BM. An anchoring role for FeS Clusters: Chelation of the amino acid moiety of S-adenosylmethionine to the unique iron site of the [4Fe-4S] cluster of pyruvate formate-lyase activating enzyme. J Am Chem Soc. 2002;124:11270. - PubMed
- Chen D, Walsby C, Hoffman BM, Frey PA. Coordination and mechanism of reversible cleavage of S-adenosylmethionine by the [4Fe-4S] center in lysine 2,3-aminomutase. J Am Chem Soc. 2003;125:11788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM054608/GM/NIGMS NIH HHS/United States
- GM 54608/GM/NIGMS NIH HHS/United States
- GM 111097/GM/NIGMS NIH HHS/United States
- R01 GM111097/GM/NIGMS NIH HHS/United States
- R29 GM054608/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases