Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood - PubMed (original) (raw)
. 2017 Sep;22(5):1191-1204.
doi: 10.1111/adb.12404. Epub 2016 May 15.
Affiliations
- PMID: 27183824
- PMCID: PMC5110402
- DOI: 10.1111/adb.12404
Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood
Evan J Kyzar et al. Addict Biol. 2017 Sep.
Abstract
Alcohol exposure in adolescence is an important risk factor for the development of alcoholism in adulthood. Epigenetic processes are implicated in the persistence of adolescent alcohol exposure-related changes, specifically in the amygdala. We investigated the role of histone methylation mechanisms in the persistent effects of adolescent intermittent ethanol (AIE) exposure in adulthood. Adolescent rats were exposed to 2 g/kg ethanol (2 days on/off) or intermittent n-saline (AIS) during postnatal days (PND) 28-41 and used for behavioral and epigenetic studies. We found that AIE exposure caused a long-lasting decrease in mRNA and protein levels of lysine demethylase 1(Lsd1) and mRNA levels of Lsd1 + 8a (a neuron-specific splice variant) in specific amygdaloid structures compared with AIS-exposed rats when measured at adulthood. Interestingly, AIE increased histone H3 lysine 9 dimethylation (H3K9me2) levels in the central nucleus of the amygdala (CeA) and medial nucleus of the amygdala (MeA) in adulthood without producing any change in H3K4me2 protein levels. Acute ethanol challenge (2 g/kg) in adulthood attenuated anxiety-like behaviors and the decrease in Lsd1 + 8a mRNA levels in the amygdala induced by AIE. AIE caused an increase in H3K9me2 occupancy at the brain-derived neurotrophic factor exon IV promoter in the amygdala that returned to baseline after acute ethanol challenge in adulthood. These results indicate that AIE specifically modulates epizymes involved in H3K9 dimethylation in the amygdala in adulthood, which are possibly responsible for AIE-induced chromatin remodeling and adult psychopathology such as anxiety.
Keywords: alcohol; amygdala; anxiety; epigenetics; histone demethylase; histone methylation; lysine demethylase 1.
© Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
Figures
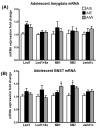
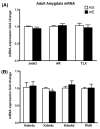
Figure 1. mRNA profiling of histone methyltransferases and demethylases in the amygdala (A) and BNST (B) of adolescent rats exposed to AIE
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and their brains were collected and amygdaloid as well as BNST tissues were dissected out for mRNA analysis at postnatal day (PND) 41-42 either 1 hr after last ethanol exposure (AIE group) or 24 hrs after last ethanol exposure during withdrawal (AIW group) in the amygdala (A) and BNST (B). Values are represented as mean ± SEM of n=6-7 rats and are significantly different from AIS-exposed controls (*p < 0.05, **p<0.01, one-way ANOVA followed by post hoc Tukey's test).
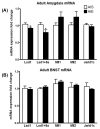
Figure 2. mRNA profiling of histone methyltransferases and demethylases in the amygdala (A) and BNST (B) of adult rats previously exposed to AIE
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and allowed to grow to adulthood without further ethanol or saline exposure. Their brains were collected and amygdaloid as well as BNST tissues were dissected out for mRNA analysis at postnatal day (PND) 92. Values are represented as mean ± SEM of n=5-8 rats and are significantly different from AIS-exposed group (*p < 0.05, Student's t test).
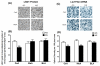
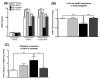
Figure 3. Decreased LSD1 protein and Lsd1+8a mRNA in specific amygdaloid structures of adult rats exposed to AIE
A) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of LSD1 in the central (CeA) and medial nucleus of amygdala (MeA) of adolescent intermittent ethanol (AIE) - or saline (AIS)-exposed adult rats. B) Quantification of LSD1 protein levels by gold immunolabeling in the CeA, MeA and basolateral amygdala (BLA) of AIS- and AIE-exposed adult rats. C) Photomicrographs (Scale bar = 50 μm) showing the Lsd1+8a mRNA positive cells (in-situ PCR) in the CeA and MeA of AIE- and AIS-exposed adult rats. D) Quantification of Lsd1+8a mRNA levels by in situ PCR in the CeA, MeA and BLA of AIS- and AIE-exposed adult rats. Values are represented as mean ± SEM of n=5 rats and are significantly different from AIS-exposed group (**p < 0.01, ***p < 0.001, Student's t test).
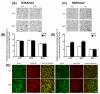
Figure 4. Increased histone H3 lysine 9 dimethlyation (H3K9me2) but no change in H3K4me2 protein levels in the amygdala of AIE adult rats
A) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of H3K4me2 proteins in the central (CeA) and medial nucleus of amygdala (MeA) of adolescent intermittent ethanol (AIE)- or saline (AIS)-exposed adult rats. B) Quantification of H3K4me2 protein levels by gold immunolabeling in the CeA, MeA and basolateral amygdala (BLA) of AIS- and AIE-exposed adult rats. C) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of H3K9me2 proteins in the CeA and MeA of AIE- and AIS-exposed adult rats. D) Quantification of H3K9me2 protein levels by gold immunolabeling in the CeA, MeA and BLA of AIS- and AIE-exposed adult rats. Values are represented as mean ± SEM of n=5 rats and are significantly different from AIS-exposed group (***p < 0.001, Student's t test). E) Representative confocal photomicrographs of immunefluorescent staining (Scale bar = 50 μm) of H3K9me2 (red) co-localized with either neuron-specific nuclear protein (NeuN; green) or glial fibrillary acidic protein (GFAP; green) in the CeA and MeA of naive adult rats.
Figure 5. mRNA profiling of (A) _Lsd1_-interacting partners and (B) histone H3 lysine 9 (H3K9) methylation modifying enzymes reveals a specific decrease in Kdm4c in the amygdala of AIE adult rats
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and allowed to grow to adulthood without further ethanol exposure. Their brains were collected and amygdaloid tissues dissected out for mRNA analysis at postnatal day (PND) 92. Values are represented as mean ± SEM of n=5-9 rats and are significantly different from AIS-exposed group (*p < 0.05, Student's t test). AR, androgen receptor; TLX, nuclear receptor.
Figure 6. Acute ethanol challenge normalizes (A) anxiety-like behavior, (B) amygdala Lsd1+8a expression, and (C) amygdala histone H3 lysine 9 dimethylation (H3K9me2) occupancy at Bdnf exon IV promoter
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) in adolescence (postnatal day [PND] 28-41) and no further exposure to ethanol until animals were exposed to an acute ethanol challenge (2 g/kg) at PND 101 or 102. Animals were tested in the elevated plus maze (EPM) for anxiety-like behaviors (activities in open and closed arms), and immediately brains were collected and amygdaloid tissues dissected out for mRNA and chromatin immunoprecipitation (ChIP) analysis. Values are represented as mean ± SEM of n=6-8 rats and are significantly different between groups (*p < 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA followed by post hoc Tukey's test).
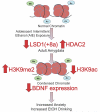
Figure 7
Hypothetical model showing adolescent intermittent ethanol (AIE) exposure causes a lasting decrease in lysine demethylase 1(LSD1) in the central nucleus of amygdala (CeA) and a decrease in Lsd1+8a in the CeA and medial nucleus of amygdala (MeA) in adulthood. Adolescent intermittent ethanol (AIE)-exposed adults show increased histone H3 lysine 9 dimethylation (H3K9me2) in the CeA and MeA with no change in histone H3 lysine 4 dimethylation (H3K4me2) levels. In addition, AIE increased the levels of H3K9me2 at the promoter of Bdnf exon IV in the amygdala. This, along with previous data from our lab showing an increase in histone deacetylase isoform 2 (HDAC2) and the resulting decrease in global and Bdnf exon IV-specific H3K9 acetylation and associated reduced levels of BDNF in the amygdala (Pandey et al., 2015) likely contributes to condensed chromatin architecture and increased anxiety-like and alcohol-drinking behaviors.
Similar articles
- MicroRNA-137 Drives Epigenetic Reprogramming in the Adult Amygdala and Behavioral Changes after Adolescent Alcohol Exposure.
Kyzar EJ, Bohnsack JP, Zhang H, Pandey SC. Kyzar EJ, et al. eNeuro. 2019 Dec 3;6(6):ENEURO.0401-19.2019. doi: 10.1523/ENEURO.0401-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31740576 Free PMC article. - Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Adolescent Alcohol Exposure-Induced Changes in Alpha-Melanocyte Stimulating Hormone and Neuropeptide Y Pathways via Histone Acetylation in the Brain During Adulthood.
Kokare DM, Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC. Kokare DM, et al. Int J Neuropsychopharmacol. 2017 Sep 1;20(9):758-768. doi: 10.1093/ijnp/pyx041. Int J Neuropsychopharmacol. 2017. PMID: 28575455 Free PMC article. - Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.
Godino A, Jayanthi S, Cadet JL. Godino A, et al. Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review. - [Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].
Legastelois R, Jeanblanc J, Vilpoux C, Bourguet E, Naassila M. Legastelois R, et al. Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
Cited by
- Adolescent binge ethanol impacts H3K36me3 regulation of synaptic genes.
Brocato ER, Wolstenholme JT. Brocato ER, et al. Front Mol Neurosci. 2023 Mar 3;16:1082104. doi: 10.3389/fnmol.2023.1082104. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36937047 Free PMC article. - Acute Ethanol Produces Ataxia and Induces Fmr1 Expression via Histone Modifications in the Rat Cerebellum.
Dulman RS, Auta J, Teppen T, Pandey SC. Dulman RS, et al. Alcohol Clin Exp Res. 2019 Jun;43(6):1191-1198. doi: 10.1111/acer.14044. Epub 2019 May 14. Alcohol Clin Exp Res. 2019. PMID: 30969437 Free PMC article. - Altered amygdala DNA methylation mechanisms after adolescent alcohol exposure contribute to adult anxiety and alcohol drinking.
Sakharkar AJ, Kyzar EJ, Gavin DP, Zhang H, Chen Y, Krishnan HR, Grayson DR, Pandey SC. Sakharkar AJ, et al. Neuropharmacology. 2019 Oct;157:107679. doi: 10.1016/j.neuropharm.2019.107679. Epub 2019 Jun 20. Neuropharmacology. 2019. PMID: 31229451 Free PMC article. - Epigenetic mechanisms of alcoholism and stress-related disorders.
Palmisano M, Pandey SC. Palmisano M, et al. Alcohol. 2017 May;60:7-18. doi: 10.1016/j.alcohol.2017.01.001. Epub 2017 Mar 3. Alcohol. 2017. PMID: 28477725 Free PMC article. Review. - Changes in Neuroimmune and Neuronal Death Markers after Adolescent Alcohol Exposure in Rats are Reversed by Donepezil.
Swartzwelder HS, Healey KL, Liu W, Dubester K, Miller KM, Crews FT. Swartzwelder HS, et al. Sci Rep. 2019 Aug 20;9(1):12110. doi: 10.1038/s41598-019-47039-1. Sci Rep. 2019. PMID: 31431637 Free PMC article.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. - PubMed
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. - PubMed
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. - PubMed
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addict Behav. 2001;26:803–825. - PubMed
MeSH terms
Substances
Grants and funding
- I01 BX000143/BX/BLRD VA/United States
- U01 AA019971/AA/NIAAA NIH HHS/United States
- VA999999/Intramural VA/United States
- F30 AA024948/AA/NIAAA NIH HHS/United States
- R01 AA010005/AA/NIAAA NIH HHS/United States
- U24 AA024605/AA/NIAAA NIH HHS/United States
- P50 AA022538/AA/NIAAA NIH HHS/United States
- R01 AA013341/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources