Mutations in pepQ Confer Low-Level Resistance to Bedaquiline and Clofazimine in Mycobacterium tuberculosis - PubMed (original) (raw)
Mutations in pepQ Confer Low-Level Resistance to Bedaquiline and Clofazimine in Mycobacterium tuberculosis
Deepak Almeida et al. Antimicrob Agents Chemother. 2016.
Abstract
The novel ATP synthase inhibitor bedaquiline recently received accelerated approval for treatment of multidrug-resistant tuberculosis and is currently being studied as a component of novel treatment-shortening regimens for drug-susceptible and multidrug-resistant tuberculosis. In a limited number of bedaquiline-treated patients reported to date, ≥4-fold upward shifts in bedaquiline MIC during treatment have been attributed to non-target-based mutations in Rv0678 that putatively increase bedaquiline efflux through the MmpS5-MmpL5 pump. These mutations also confer low-level clofazimine resistance, presumably by a similar mechanism. Here, we describe a new non-target-based determinant of low-level bedaquiline and clofazimine cross-resistance in Mycobacterium tuberculosis: loss-of-function mutations in pepQ (Rv2535c), which corresponds to a putative Xaa-Pro aminopeptidase. pepQ mutants were selected in mice by treatment with clinically relevant doses of bedaquiline, with or without clofazimine, and were shown to have bedaquiline and clofazimine MICs 4 times higher than those for the parental H37Rv strain. Coincubation with efflux inhibitors verapamil and reserpine lowered bedaquiline MICs against both mutant and parent strains to a level below the MIC against H37Rv in the absence of efflux pump inhibitors. However, quantitative PCR (qPCR) revealed no significant differences in expression of Rv0678, mmpS5, or mmpL5 between mutant and parent strains. Complementation of a pepQ mutant with the wild-type gene restored susceptibility, indicating that loss of PepQ function is sufficient for reduced susceptibility both in vitro and in mice. Although the mechanism by which mutations in pepQ confer bedaquiline and clofazimine cross-resistance remains unclear, these results may have clinical implications and warrant further evaluation of clinical isolates with reduced susceptibility to either drug for mutations in this gene.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
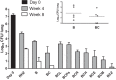
FIG 1
Mean lung log10 CFU counts (±SD) after 4 and 8 weeks of treatment with bedaquiline- and clofazimine-containing regimens. The main graph does not include results from mice in which bedaquiline and clofazimine cross-resistant mutants were selected after 8 weeks of treatment: 2 and 3 mice from the B and BC groups with mean lung CFU counts of 3.22 ± 0.16 and 2.12 ± 0.69 log10, respectively (inset). The CFU counts at the time of treatment initiation are indicated at day 0. Abbreviations: R, rifampin; H, isoniazid; Z, pyrazinamide; B, bedaquiline; C, clofazimine; L, linezolid; Pa, pretomanid; E, ethambutol; M, moxifloxacin.
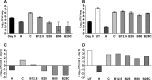
FIG 2
Mean lung log10 CFU counts (±SD) (A and B) and change in CFU counts (week 4 − day 0) (C and D) after 4 weeks of treatment in mice infected with the parental H37Rv strain (A and C) or the B5 mutant (B and D). CFU counts were not determined for untreated mice infected with the parental strain, which required euthanasia prior to the predetermined endpoint. Abbreviations: UT, untreated; H, isoniazid (10 mg/kg); C, clofazimine (20 mg/kg); B, bedaquiline (12.5, 25, or 50 mg/kg).
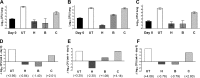
FIG 3
Mean lung log10 CFU counts (±SD) (A to C) and change in lung CFU counts (week 4 − day 0) (D to F) after 4 weeks of treatment in mice infected with the parental H37Rv strain (A and D), the B5 mutant (B and E), or the complemented B5::pepQ strain (C and F). Abbreviations: UT, untreated; H, isoniazid (10 mg/kg); C, clofazimine (20 mg/kg); B, bedaquiline (25 mg/kg).
FIG 4
Locations of mutations and domains in pepQ.
FIG 5
Multiple amino acid sequence alignment for the products of M. tuberculosis pepQ, Lactococcus lactis pepP, and E. coli pepP, constructed using ClustalW. Residues coordinating the Mn2+ ions are shown in red. Asterisks indicate positions which have a single, fully conserved residue. Colons indicate conservation between groups of strongly similar properties, scoring >0.5 in the Gonnet PAM 250 matrix. Periods indicate conservation between groups of weakly similar properties, scoring ≤0.5 in the Gonnet PAM 250 matrix.
Similar articles
- Bedquiline Resistance Mutations: Correlations with Drug Exposures and Impact on the Proteome in M. tuberculosis.
Xu J, Li D, Shi J, Wang B, Ge F, Guo Z, Mu X, Nuermberger E, Lu Y. Xu J, et al. Antimicrob Agents Chemother. 2023 Jul 18;67(7):e0153222. doi: 10.1128/aac.01532-22. Epub 2023 May 31. Antimicrob Agents Chemother. 2023. PMID: 37255473 Free PMC article. - Population-level emergence of bedaquiline and clofazimine resistance-associated variants among patients with drug-resistant tuberculosis in southern Africa: a phenotypic and phylogenetic analysis.
Nimmo C, Millard J, van Dorp L, Brien K, Moodley S, Wolf A, Grant AD, Padayatchi N, Pym AS, Balloux F, O'Donnell M. Nimmo C, et al. Lancet Microbe. 2020 Aug;1(4):e165-e174. doi: 10.1016/S2666-5247(20)30031-8. Lancet Microbe. 2020. PMID: 32803174 Free PMC article. - Bedaquiline- and clofazimine- selected Mycobacterium tuberculosis mutants: further insights on resistance driven largely by Rv0678.
Snobre J, Villellas MC, Coeck N, Mulders W, Tzfadia O, de Jong BC, Andries K, Rigouts L. Snobre J, et al. Sci Rep. 2023 Jun 27;13(1):10444. doi: 10.1038/s41598-023-36955-y. Sci Rep. 2023. PMID: 37369740 Free PMC article. - Bedaquiline: a novel antitubercular agent for the treatment of multidrug-resistant tuberculosis.
Worley MV, Estrada SJ. Worley MV, et al. Pharmacotherapy. 2014 Nov;34(11):1187-97. doi: 10.1002/phar.1482. Epub 2014 Sep 9. Pharmacotherapy. 2014. PMID: 25203970 Review. - Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis.
Chahine EB, Karaoui LR, Mansour H. Chahine EB, et al. Ann Pharmacother. 2014 Jan;48(1):107-15. doi: 10.1177/1060028013504087. Epub 2013 Nov 1. Ann Pharmacother. 2014. PMID: 24259600 Review.
Cited by
- Molecular mechanisms of resistance and treatment efficacy of clofazimine and bedaquiline against Mycobacterium tuberculosis.
Islam MM, Alam MS, Liu Z, Khatun MS, Yusuf B, Hameed HMA, Tian X, Chhotaray C, Basnet R, Abraha H, Zhang X, Khan SA, Fang C, Li C, Hasan S, Tan S, Zhong N, Hu J, Zhang T. Islam MM, et al. Front Med (Lausanne). 2024 Jan 10;10:1304857. doi: 10.3389/fmed.2023.1304857. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38274444 Free PMC article. Review. - Empirical ways to identify novel Bedaquiline resistance mutations in AtpE.
Karmakar M, Rodrigues CHM, Holt KE, Dunstan SJ, Denholm J, Ascher DB. Karmakar M, et al. PLoS One. 2019 May 29;14(5):e0217169. doi: 10.1371/journal.pone.0217169. eCollection 2019. PLoS One. 2019. PMID: 31141524 Free PMC article. - Bedaquiline Resistance and Molecular Characterization of Rifampicin-Resistant Mycobacterium Tuberculosis Isolates in Zhejiang, China.
Tong E, Zhou Y, Liu Z, Zhu Y, Zhang M, Wu K, Pan J, Jiang J. Tong E, et al. Infect Drug Resist. 2023 Oct 31;16:6951-6963. doi: 10.2147/IDR.S429003. eCollection 2023. Infect Drug Resist. 2023. PMID: 37928607 Free PMC article. - Validation of Bedaquiline Phenotypic Drug Susceptibility Testing Methods and Breakpoints: a Multilaboratory, Multicountry Study.
Kaniga K, Aono A, Borroni E, Cirillo DM, Desmaretz C, Hasan R, Joseph L, Mitarai S, Shakoor S, Torrea G, Ismail NA, Omar SV. Kaniga K, et al. J Clin Microbiol. 2020 Mar 25;58(4):e01677-19. doi: 10.1128/JCM.01677-19. Print 2020 Mar 25. J Clin Microbiol. 2020. PMID: 31969421 Free PMC article. - Emergence of mmpT5 Variants during Bedaquiline Treatment of Mycobacterium intracellulare Lung Disease.
Alexander DC, Vasireddy R, Vasireddy S, Philley JV, Brown-Elliott BA, Perry BJ, Griffith DE, Benwill JL, Cameron AD, Wallace RJ Jr. Alexander DC, et al. J Clin Microbiol. 2017 Feb;55(2):574-584. doi: 10.1128/JCM.02087-16. Epub 2016 Dec 7. J Clin Microbiol. 2017. PMID: 27927925 Free PMC article.
References
- Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi:10.1126/science.1106753. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources