Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99 - PubMed (original) (raw)
Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99
Lora G Bankova et al. Proc Natl Acad Sci U S A. 2016.
Abstract
Cysteinyl leukotrienes (cysLTs), leukotriene C4 (LTC4), LTD4, and LTE4 are proinflammatory lipid mediators with pathobiologic function in asthma. LTE4, the stable cysLT, is a weak agonist for the type 1 and type 2 cysLT receptors (CysLTRs), which constrict airway smooth muscle, but elicits airflow obstruction and pulmonary inflammation in patients with asthma. We recently identified GPR99 as a high-affinity receptor for LTE4 that mediates cutaneous vascular permeability. Here we demonstrate that a single intranasal exposure to extract from the respiratory pathogen Alternaria alternata elicits profound epithelial cell (EpC) mucin release and submucosal swelling in the nasal mucosa of mice that depends on cysLTs, as it is absent in mice deficient in the terminal enzyme for cysLT biosynthesis, LTC4 synthase (LTC4S). These mucosal changes are associated with mast cell (MC) activation and absent in MC-deficient mice, suggesting a role for MCs in control of EpC function. Of the three CysLTRs, only GPR99-deficient mice are fully protected from EpC mucin release and swelling elicited by Alternaria or by intranasal LTE4 GPR99 expression is detected on lung and nasal EpCs, which release mucin to doses of LTE4 one log lower than that required to elicit submucosal swelling. Finally, mice deficient in MCs, LTC4S, or GPR99 have reduced baseline numbers of goblet cells, indicating an additional function in regulating EpC homeostasis. These results demonstrate a novel role for GPR99 among CysLTRs in control of respiratory EpC function and suggest that inhibition of LTE4 and of GPR99 may have therapeutic benefits in asthma.
Keywords: cysteinyl leukotrienes; epithelial cell; lung; mast cells; mucosal immunology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
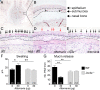
cysLT-dependent submucosal swelling and mucin release elicited by Alternaria. (A) Representative histological coronal section through the incisors of the mouse skull of a PBS-challenged mouse with CAE stain (50× magnification). (B) A 630× magnification of Inset in A, rotated 90°. Black arrowheads point to MCs. (C_–_E) PAS staining of nasal septum 1 h after i.n. administration of (C) PBS or (D and E) 30 µg of Alternaria (magnification: 630×). (Scale bar, 20 µm.) Black arrows indicate mucin-containing GCs. Red arrows indicate GCs with partial extrusion of mucin. (F and G) WT (black bars) and Ltc4s −/− (gray bars) mice. (F) Submucosal swelling, indicated by the difference in the submucosal area between PBS (0 µg) and _Alternaria_-treated (30 µg) mice. (G) Mucin release, indicated by the difference in the total number of PAS+ cells detected between PBS and _Alternaria_-treated mice. Results are means ± SEM pooled from four independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01.
Fig. 2.
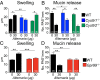
The innate activation of MCs is required for _Alternaria-_elicited mucosal responses. (A) Percentage of degranulating MCs in the nasal submucosa 1 h after i.n. administration of PBS (0 µg) or Alternaria (30 µg) to WT (black bars) and Fcer1g ‒/‒ (brown bars) mice. (B) ELISA quantification of PGD2 in the NL 1 h after i.n. administration of PBS or Alternaria to WT, Fcer1g ‒/‒, and Mcpt5/DTA (white bars) mice. (C) BMMCs from WT, Fcer1g ‒/‒, or Ltc4s ‒/‒ mice were harvested after 5–8 wk of culture and stimulated with Alternaria. The concentrations of cysLTs in the supernatants were measured by enzyme immunoassay at 30 min. Results are means ± SEM pooled from three independent experiments with cultures obtained from 10 WT mice, 3 Ltc4s ‒/‒ mice, and 3 Fcer1g ‒/‒ mice. (D) β-Hexosaminidase release into the supernatant at 30 min as a percentage of total. Results are means ± SEM pooled from three independent BMMC cultures and three independent human CD34+ cell-derived MC cultures. (E) Submucosal swelling and (F) mucin release in WT, Mcpt5/DTA, and Fcer1g ‒/‒ mice 1 h after a single dose of PBS or Alternaria. Results are means ± SEM pooled from three (A) or five (B, E, and F) independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S1.
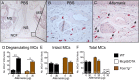
MCs in the nasal cavity degranulate in response to Alternaria. (A) Representative histological coronal section through the first molar teeth of the mouse skull of a PBS-challenged mouse with CAE stain. MS, maxillary sinuses; NCF, nasal cavity floor; NS, nasal septum (50× magnification). (B) Magnification of Inset from A; CAE-reactive MCs stain red. (C) Degranulating MCs in the nasal cavity floor 1 h after Alternaria administration. Arrows point to degranulating MCs (magnification: 630×). (D) Number of degranulating MCs. (E) Number of intact nondegranulating MCs. (F) Total number of MCs (degranulated + intact MCs) enumerated in seven 200× fields in WT (black bars), Fcer1g ‒/‒ (brown bars), and Mcpt5/DTA (open bars) mice. Data are means ± SEM pooled from three to four independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01.
Fig. 3.
GPR99 controls _Alternaria_-elicited EpC mucin release and baseline EpC composition. (A_–_D) Alternaria (30 µg) or PBS (0 µg) was administered to WT (black bars), Cysltr1 ‒/‒ (blue bars), Cysltr2 ‒/‒ (green bars), and Gpr99 ‒/‒ (red bars) mice. (A and C) Swelling and (B and D) mucin release were measured in histological samples from the nasal mucosa 1 h after i.n. challenge, as in Fig. 1. Results are means ± SEM pooled from three independent experiments with the numbers of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S2.
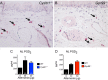
MCs in the nasal cavity of Cysltr1 ‒/‒ and Gpr99 ‒/‒ mice are activated after i.n. Alternaria administration. (A and B) Degranulating MCs in the nasal submucosa of (A) Cysltr1 ‒/‒ and (B) Gpr99 ‒/‒ mice. Magnification: 400×. Arrows indicate degranulating MCs. (C and D) PGD2 in the NL of WT (black bars), Cysltr1 ‒/‒ (blue bars), and Gpr99 ‒/‒ (red bars) mice. Data are means ± SEM from one experiment with the number of mice per group indicated on each bar in parentheses.
Fig. 4.
GPR99 is expressed in respiratory EpCs and mediates LTE4-elicited submucosal swelling and mucin release. (A) Quantitative RT-PCR analysis of expression of Gpr99 and β-galactosidase (Bgal) genes in the nasal cavity floor (Upper panels) and nasal mucosa of the septum and side wall (Lower panels) compared with 18S rRNA. Results are means ± SEM pooled from two independent experiments with the numbers of mice per group indicated in parentheses in each column. (B and C) X-gal staining in the nasal mucosa of Gpr99 ‒/‒ mice (Right panels) and WT mice (Left panels) in the (B) nasal cavity (magnification: 630×) and (C) large bronchi (magnification: 200×). Positive X-gal reactivity produces a blue precipitate. (D and E) LTE4 (0.01, 0.1, or 1 nmol) or PBS (0 nmol) was administered i.n. to WT (black bars), Cysltr1 ‒/‒ (blue bars), and Gpr99 ‒/‒ (red bars) mice. (D) Submucosal swelling and (E) mucin release were measured 1 h after LTE4 administration in histological samples from the nasal mucosa. Data are means ± SEM pooled from three independent experiments with the numbers of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fig. S3.
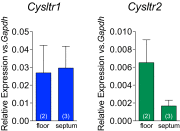
Quantitative RT-PCR analysis of CysLT1R (blue bars) and CysLT2R (green bars) expression in the nasal cavity floor (“floor”) and nasal septum (“septum”) of WT mice. Data are means ± SEM from one experiment with number of mice per group indicated on each bar in parentheses.
Fig. S4.
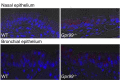
Confocal microscopy of nasal (Upper panels) and bronchial (Lower panels) epithelia stained with X-gal. The X-gal precipitate fluoresces in the far red spectrum. Nuclei stained blue with Hoechst 33342 solution. Magnification: 630×.
Similar articles
- Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand.
Kanaoka Y, Maekawa A, Austen KF. Kanaoka Y, et al. J Biol Chem. 2013 Apr 19;288(16):10967-72. doi: 10.1074/jbc.C113.453704. Epub 2013 Mar 15. J Biol Chem. 2013. PMID: 23504326 Free PMC article. - Leukotriene E4 induces MUC5AC release from human airway epithelial NCI-H292 cells.
Shirasaki H, Kanaizumi E, Seki N, Himi T. Shirasaki H, et al. Allergol Int. 2015 Apr;64(2):169-74. doi: 10.1016/j.alit.2014.11.002. Epub 2015 Jan 13. Allergol Int. 2015. PMID: 25838093 - Type 2 Cysteinyl Leukotriene Receptors Drive IL-33-Dependent Type 2 Immunopathology and Aspirin Sensitivity.
Liu T, Barrett NA, Kanaoka Y, Yoshimoto E, Garofalo D, Cirka H, Feng C, Boyce JA. Liu T, et al. J Immunol. 2018 Feb 1;200(3):915-927. doi: 10.4049/jimmunol.1700603. Epub 2017 Dec 27. J Immunol. 2018. PMID: 29282304 Free PMC article. - Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions.
Kanaoka Y, Austen KF. Kanaoka Y, et al. Adv Immunol. 2019;142:65-84. doi: 10.1016/bs.ai.2019.04.002. Epub 2019 May 14. Adv Immunol. 2019. PMID: 31296303 Review. - Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights.
Elieh Ali Komi D, Bjermer L. Elieh Ali Komi D, et al. Clin Rev Allergy Immunol. 2019 Apr;56(2):234-247. doi: 10.1007/s12016-018-8720-1. Clin Rev Allergy Immunol. 2019. PMID: 30506113 Review.
Cited by
- A scoping review: What are the cellular mechanisms that drive the allergic inflammatory response to fungal allergens in the lung epithelium?
Goode EJ, Marczylo E. Goode EJ, et al. Clin Transl Allergy. 2023 Jun;13(6):e12252. doi: 10.1002/clt2.12252. Clin Transl Allergy. 2023. PMID: 37357550 Free PMC article. Review. - Anti-Allergic Effect of Dietary Polyphenols Curcumin and Epigallocatechin Gallate via Anti-Degranulation in IgE/Antigen-Stimulated Mast Cell Model: A Lipidomics Perspective.
Zeng J, Hao J, Yang Z, Ma C, Gao L, Chen Y, Li G, Li J. Zeng J, et al. Metabolites. 2023 May 5;13(5):628. doi: 10.3390/metabo13050628. Metabolites. 2023. PMID: 37233669 Free PMC article. - Efficacy and safety of montelukast adjuvant therapy in adults with cough variant asthma: A systematic review and meta-analysis.
Xu Q, Lu T, Song Z, Zhu P, Wu Y, Zhang L, Yang K, Zhang Z. Xu Q, et al. Clin Respir J. 2023 Oct;17(10):986-997. doi: 10.1111/crj.13629. Epub 2023 May 22. Clin Respir J. 2023. PMID: 37218346 Free PMC article. Review. - The immunometabolite itaconate stimulates OXGR1 to promote mucociliary clearance during the pulmonary innate immune response.
Zeng YR, Song JB, Wang D, Huang ZX, Zhang C, Sun YP, Shu G, Xiong Y, Guan KL, Ye D, Wang P. Zeng YR, et al. J Clin Invest. 2023 Mar 15;133(6):e160463. doi: 10.1172/JCI160463. J Clin Invest. 2023. PMID: 36919698 Free PMC article. - Basophils from allergy to cancer.
Poto R, Gambardella AR, Marone G, Schroeder JT, Mattei F, Schiavoni G, Varricchi G. Poto R, et al. Front Immunol. 2022 Dec 12;13:1056838. doi: 10.3389/fimmu.2022.1056838. eCollection 2022. Front Immunol. 2022. PMID: 36578500 Free PMC article. Review.
References
- Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142(1):112–119. - PubMed
- Christie PE, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–1029. - PubMed
- Drazen JM, et al. Recovery of leukotriene E4 from the urine of patients with airway obstruction. Am Rev Respir Dis. 1992;146(1):104–108. - PubMed
- Lynch KR, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789–793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous