RECIST 1.1-Update and clarification: From the RECIST committee - PubMed (original) (raw)
doi: 10.1016/j.ejca.2016.03.081. Epub 2016 May 14.
Saskia Litière 2, Elisabeth de Vries 3, Robert Ford 4, Stephen Gwyther 5, Sumithra Mandrekar 6, Lalitha Shankar 7, Jan Bogaerts 2, Alice Chen 8, Janet Dancey 9, Wendy Hayes 10, F Stephen Hodi 11, Otto S Hoekstra 12, Erich P Huang 13, Nancy Lin 11, Yan Liu 2, Patrick Therasse 14, Jedd D Wolchok 15, Lesley Seymour 9
Affiliations
- PMID: 27189322
- PMCID: PMC5737828
- DOI: 10.1016/j.ejca.2016.03.081
RECIST 1.1-Update and clarification: From the RECIST committee
Lawrence H Schwartz et al. Eur J Cancer. 2016 Jul.
Abstract
The Response Evaluation Criteria in Solid Tumours (RECIST) were developed and published in 2000, based on the original World Health Organisation guidelines first published in 1981. In 2009, revisions were made (RECIST 1.1) incorporating major changes, including a reduction in the number of lesions to be assessed, a new measurement method to classify lymph nodes as pathologic or normal, the clarification of the requirement to confirm a complete response or partial response and new methodologies for more appropriate measurement of disease progression. The purpose of this paper was to summarise the questions posed and the clarifications provided as an update to the 2009 publication.
Keywords: Clarifications; RECIST; Tumour response.
Copyright © 2016. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement: Author and all other co-authors: none relevant
Figures
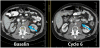
Fig 1
Note optimal manner for measuring the short axis of coalescing lymph nodes (blue arrow). At baseline, there are two distinct nodes, therefore the short axis is measured for each (red and yellow lines) and at cycle 6 the single short axis of the coalesced node is now measured as a single line (red).
Fig. 2
Patient with colorectal cancer. Liver metastases at baseline (red circle) appear to resolved at cycle 3 with “reappearance” at cycle 5 (red circle). However, notice that the imaging techniques are different and the cycle 3 is of poor image quality to visualize these metastases. Therefore, these lesions have not truly reappeared and the patient should not be considered to have progressive disease at cycle 5. There is true progression at cycle 7.
Similar articles
- New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. Eisenhauer EA, et al. Eur J Cancer. 2009 Jan;45(2):228-47. doi: 10.1016/j.ejca.2008.10.026. Eur J Cancer. 2009. PMID: 19097774 - Evaluation of lymph nodes with RECIST 1.1.
Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, Eisenhauer EA. Schwartz LH, et al. Eur J Cancer. 2009 Jan;45(2):261-7. doi: 10.1016/j.ejca.2008.10.028. Epub 2008 Dec 16. Eur J Cancer. 2009. PMID: 19091550 - RECIST 1.1 - Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group.
Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, de Vries E. Schwartz LH, et al. Eur J Cancer. 2016 Jul;62:138-45. doi: 10.1016/j.ejca.2016.03.082. Epub 2016 May 26. Eur J Cancer. 2016. PMID: 27237360 Free PMC article. Review. - Response evaluation for immunotherapy through semi-automatic software based on RECIST 1.1, irRC, and iRECIST criteria: comparison with subjective assessment.
Lai YC, Chang WC, Chen CB, Wang CL, Lin YF, Ho MM, Cheng CY, Huang PW, Hsu CW, Lin G. Lai YC, et al. Acta Radiol. 2020 Jul;61(7):983-991. doi: 10.1177/0284185119887588. Epub 2019 Nov 18. Acta Radiol. 2020. PMID: 31739675 - RECIST - learning from the past to build the future.
Litière S, Collette S, de Vries EG, Seymour L, Bogaerts J. Litière S, et al. Nat Rev Clin Oncol. 2017 Mar;14(3):187-192. doi: 10.1038/nrclinonc.2016.195. Epub 2016 Dec 20. Nat Rev Clin Oncol. 2017. PMID: 27995946 Review.
Cited by
- Imaging and liquid biopsy in the prediction and evaluation of response to PRRT in neuroendocrine tumors: implications for patient management.
Roll W, Weckesser M, Seifert R, Bodei L, Rahbar K. Roll W, et al. Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):4016-4027. doi: 10.1007/s00259-021-05359-3. Epub 2021 Apr 26. Eur J Nucl Med Mol Imaging. 2021. PMID: 33903926 Free PMC article. Review. - Enhancing nasopharyngeal carcinoma cell radiosensitivity by suppressing AKT/mTOR via CENP-N knockdown.
Wu LZ, Zou Y, Wang BR, Ni HF, Kong YG, Hua QQ, Chen SM. Wu LZ, et al. J Transl Med. 2023 Nov 8;21(1):792. doi: 10.1186/s12967-023-04654-x. J Transl Med. 2023. PMID: 37940975 Free PMC article. - PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients.
Kim JW, Lim AR, You JY, Lee JH, Song SE, Lee NK, Jung SP, Cho KR, Kim CY, Park KH. Kim JW, et al. Cancer Res Treat. 2023 Apr;55(2):531-541. doi: 10.4143/crt.2022.221. Epub 2022 Sep 7. Cancer Res Treat. 2023. PMID: 36097803 Free PMC article. - A real-world study of immune checkpoint inhibitors in advanced triple-negative breast cancer.
Zhang Z, Zhang Y, Liu C, Shao J, Chen Y, Zhu Y, Zhang L, Qin B, Kong Z, Wang X, Wang Y, Huang D, Liu L, Zhou Y, Tao R, Yang Z, Liu M, Zhao W. Zhang Z, et al. Cancer Innov. 2023 Apr 23;2(3):172-180. doi: 10.1002/cai2.70. eCollection 2023 Jun. Cancer Innov. 2023. PMID: 38089401 Free PMC article.
References
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. - PubMed
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. - PubMed
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. - PubMed
- Janku F, Berry DA, Gong J, Parsons HA, Stewart DJ, Kurzrock R. Outcomes of phase II clinical trials with single-agent therapies in advanced/metastatic non–small cell lung cancer published between 2000 and 2009. Clin Cancer Res. 2012;18:6356–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources