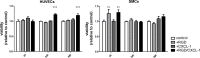Targeting In-Stent-Stenosis with RGD- and CXCL1-Coated Mini-Stents in Mice - PubMed (original) (raw)
. 2016 May 18;11(5):e0155829.
doi: 10.1371/journal.pone.0155829. eCollection 2016.
Sakine Simsekyilmaz 1 2 3, Elisa A Liehn 1 4 5, Fabian Schreiber 7, Remco T A Megens 8, Wendy Theelen 1, Ralf Smeets 9, Stefan Jockenhövel 6, Thomas Gries 7, Martin Möller 2, Doris Klee 2, Christian Weber 8, Alma Zernecke 10
Affiliations
- PMID: 27192172
- PMCID: PMC4871500
- DOI: 10.1371/journal.pone.0155829
Targeting In-Stent-Stenosis with RGD- and CXCL1-Coated Mini-Stents in Mice
Sakine Simsekyilmaz et al. PLoS One. 2016.
Abstract
Atherosclerotic lesions that critically narrow the artery can necessitate an angioplasty and stent implantation. Long-term therapeutic effects, however, are limited by excessive arterial remodeling. We here employed a miniaturized nitinol-stent coated with star-shaped polyethylenglycole (star-PEG), and evaluated its bio-functionalization with RGD and CXCL1 for improving in-stent stenosis after implantation into carotid arteries of mice. Nitinol foils or stents (bare metal) were coated with star-PEG, and bio-functionalized with RGD, or RGD/CXCL1. Cell adhesion to star-PEG-coated nitinol foils was unaltered or reduced, whereas bio-functionalization with RGD but foremost RGD/CXCL1 increased adhesion of early angiogenic outgrowth cells (EOCs) and endothelial cells but not smooth muscle cells when compared with bare metal foils. Stimulation of cells with RGD/CXCL1 furthermore increased the proliferation of EOCs. In vivo, bio-functionalization with RGD/CXCL1 significantly reduced neointima formation and thrombus formation, and increased re-endothelialization in apoE-/- carotid arteries compared with bare-metal nitinol stents, star-PEG-coated stents, and stents bio-functionalized with RGD only. Bio-functionalization of star-PEG-coated nitinol-stents with RGD/CXCL1 reduced in-stent neointima formation. By supporting the adhesion and proliferation of endothelial progenitor cells, RGD/CXCL1 coating of stents may help to accelerate endothelial repair after stent implantation, and thus may harbor the potential to limit the complication of in-stent restenosis in clinical approaches.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
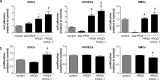
Fig 1. Biofunctionalization with RGD/CXCL1 triggers endothelial cell adhesion.
(a) Cells were seeded onto nitinol-foils (control), and nitinol-foils coated with star-PEG, or star-PEG-coated foils bio-functionalized with RGD or RGD/CXCL1. Adherent cells were quantified after 12 hours (n = 10–12). *P<0.05 vs. controls, #P<0.05 vs. star-PEG, and §P<0.05 vs. star-PEG+RGD-coated foils. One-way-ANOVA with Tukey’s multiple comparison test. (b) Cell proliferation was assessed by BrdU-incorporation (n = 9 each) after indicated stimulation, expressed relative to untreated cells (control). *P<0.05 vs. controls and #P<0.05 vs. RGD. One-way-ANOVA with Tukey’s multiple comparison test.
Fig 2. Treatment of HUVECs and SMCs with RGD and CXCL-1 does not impair cell viability.
In vitro viability assay of HUVECs and SMCs after stimulation with RGD, CXCL-1 or RGD and CXCL-1 together for 4, 24 and 48h. *P<0.05 (shown is significant difference compared to control). Two-way-ANOVA with Bonferroni post-test.
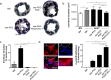
Fig 3. RGD/CXCL1-biofunctionalized stents reduce neointima formation.
(a-d) Carotid arteries of C57Bl/6 (n = 6) or _apoE_-/- mice were analyzed one week after implantation of bare metal nitinol-stents (BM, n = 4), star-PEG-coated nitinol-stents (n = 5) or star-PEG-coated stents bio-functionalized with RGD (n = 9) or RGD/CXCL1 (n = 8). (a) Representative Giemsa-stained sections (scale bars, 200μm) from _apoE_-/- mice. Quantification of in-stent intima formation (b) and thrombus formation (c). (d) Representative images of vWF-staining (red); cell nuclei were counterstained by DAPI (L, lumen; x, stent struts; scale bar, 50μm); quantification of re-endothelialization. *P<0.05. Kruskal-Wallis test with Dunn’s post-test.
Similar articles
- Polymer-free immobilization of a cyclic RGD peptide on a nitinol stent promotes integrin-dependent endothelial coverage of strut surfaces.
Joner M, Cheng Q, Schönhofer-Merl S, Lopez M, Neubauer S, Mas-Moruno C, Laufer B, Kolodgie FD, Kessler H, Virmani R. Joner M, et al. J Biomed Mater Res B Appl Biomater. 2012 Apr;100(3):637-45. doi: 10.1002/jbm.b.31988. Epub 2012 Jan 25. J Biomed Mater Res B Appl Biomater. 2012. PMID: 22278946 - Effect of a novel peptide, WKYMVm- and sirolimus-coated stent on re-endothelialization and anti-restenosis.
Jang EJ, Bae IH, Park DS, Lee SY, Lim KS, Park JK, Shim JW, Sim DS, Jeong MH. Jang EJ, et al. J Mater Sci Mater Med. 2015 Oct;26(10):251. doi: 10.1007/s10856-015-5585-1. Epub 2015 Oct 5. J Mater Sci Mater Med. 2015. PMID: 26438653 - Citric acid functionalized nitinol stent surface promotes endothelial cell healing.
Ceresnakova M, Murray D, McGourty KD, Butler J, Neilan J, Soulimane T, Hudson SP. Ceresnakova M, et al. J Biomed Mater Res A. 2021 Sep;109(9):1549-1559. doi: 10.1002/jbm.a.37150. Epub 2021 Feb 24. J Biomed Mater Res A. 2021. PMID: 33624931 - Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy.
Curcio A, Torella D, Indolfi C. Curcio A, et al. Circ J. 2011;75(6):1287-96. doi: 10.1253/circj.cj-11-0366. Epub 2011 Apr 29. Circ J. 2011. PMID: 21532177 Review. - Evolving modalities for femoropopliteal interventions.
Ansel GM, Lumsden AB. Ansel GM, et al. J Endovasc Ther. 2009 Apr;16(2 Suppl 2):II82-97. doi: 10.1583/08-2654.1. J Endovasc Ther. 2009. PMID: 19624076 Review.
Cited by
- Biofunctionalization of cardiovascular stents to induce endothelialization: Implications for in- stent thrombosis in diabetes.
Marei I, Ahmetaj-Shala B, Triggle CR. Marei I, et al. Front Pharmacol. 2022 Oct 10;13:982185. doi: 10.3389/fphar.2022.982185. eCollection 2022. Front Pharmacol. 2022. PMID: 36299902 Free PMC article. Review. - The effects of stenting on coronary endothelium from a molecular biological view: Time for improvement?
Cornelissen A, Vogt FJ. Cornelissen A, et al. J Cell Mol Med. 2019 Jan;23(1):39-46. doi: 10.1111/jcmm.13936. Epub 2018 Oct 23. J Cell Mol Med. 2019. PMID: 30353645 Free PMC article. Review. - Recent advance in treatment of atherosclerosis: Key targets and plaque-positioned delivery strategies.
Li L, Liu S, Tan J, Wei L, Wu D, Gao S, Weng Y, Chen J. Li L, et al. J Tissue Eng. 2022 Mar 24;13:20417314221088509. doi: 10.1177/20417314221088509. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 35356091 Free PMC article. Review. - Pivotal micro factors associated with endothelial cells.
Meng LB, Zhang YM, Shan MJ, Qiu Y, Zhang TJ, Gong T. Meng LB, et al. Chin Med J (Engl). 2019 Aug 20;132(16):1965-1973. doi: 10.1097/CM9.0000000000000358. Chin Med J (Engl). 2019. PMID: 31335473 Free PMC article. Review. - Biofunctional coatings and drug-coated stents for restenosis therapy.
Wen Y, Li Y, Yang R, Chen Y, Shen Y, Liu Y, Liu X, Zhang B, Li H. Wen Y, et al. Mater Today Bio. 2024 Sep 19;29:101259. doi: 10.1016/j.mtbio.2024.101259. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39391793 Free PMC article. Review.
References
- Lenzen MJ, Boersma E, Bertrand ME, Maier W, Moris C, Piscione F, et al. Management and outcome of patients with established coronary artery disease: the Euro Heart Survey on coronary revascularization. Eur Heart J. 2005; 26: 1169–1179. - PubMed
- Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012; 125: 2873–2891. 10.1161/CIRCULATIONAHA.112.097014 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This project was funded by the Deutsche Forschungsgemeinschaft (GRK 1035, ZE 827/4-1), the European Research Council Advanced Grant 249929 “Atheroprotect”, the Deutsches Zentrum für Herz-Kreislaufforschung (MHA VD1.2), the European Regional Development Fund and the state of North Rhine-Westphalia, Germany, under the Operational Program "Regional Competitiveness and Employment" 2007–2013 and the Interdisciplinary Centre for Clinical Research IZKF Aachen (junior research group to E.A.L.) within the faculty of Medicine at RWTH Aachen University.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous