Gradual conversion of cellular stress patterns into pre-stressed matrix architecture during in vitro tissue growth - PubMed (original) (raw)
Gradual conversion of cellular stress patterns into pre-stressed matrix architecture during in vitro tissue growth
Cécile M Bidan et al. J R Soc Interface. 2016 May.
Abstract
The complex arrangement of the extracellular matrix (ECM) produced by cells during tissue growth, healing and remodelling is fundamental to tissue function. In connective tissues, it is still unclear how both cells and the ECM become and remain organized over length scales much larger than the distance between neighbouring cells. While cytoskeletal forces are essential for assembly and organization of the early ECM, how these processes lead to a highly organized ECM in tissues such as osteoid is not clear. To clarify the role of cellular tension for the development of these ordered fibril architectures, we used an in vitro model system, where pre-osteoblastic cells produced ECM-rich tissue inside channels with millimetre-sized triangular cross sections in ceramic scaffolds. Our results suggest a mechanical handshake between actively contracting cells and ECM fibrils: the build-up of a long-range organization of cells and the ECM enables a gradual conversion of cell-generated tension to pre-straining the ECM fibrils, which reduces the work cells have to generate to keep mature tissue under tension.
Keywords: extracellular matrix organization; tissue growth; tissue mechanics.
© 2016 The Author(s).
Figures
Figure 1.
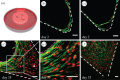
Cell organization during tissue growth in triangular millimetre-sized pores. (a) ECM-rich tissue was grown in triangular pores of HA scaffolds incubated in culture medium containing MC3T3-E1 cells. After fixation, the tissue was stained for actin (green) and nuclei (red) for fluorescent confocal imaging. Samples were fixed after 2 days (b) to reveal the elongated shape of the cells, which occasionally pulled out of the surface of the scaffold (white dashes) by the associated forces (c). Scaffolds fixed after 35 days of culture reveal the organization of the cells in the tissue at a later stage of growth and the apparition of an actin ring at the tissue–medium interface (pink dashes) (d). Throughout the culture period, cells at the tissue–medium interface have an elongated morphology (e), whereas cells embedded in the bulk spread in three dimensions (d, arrow). This transition in cell morphology as they become embedded in a three-dimensional environment also appears at the centre of a pore filled with tissue after 35 days of growth (f). Scale bar, 50 µm.
Figure 2.
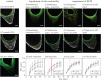
Actin and fibronectin organization during tissue growth. Fluorescent-labelled fibronectin was added to the culture medium 4 and 11 days after seeding the cells (yellow or red, respectively). Tissue was then cultured further for different periods of time, stained for actin (green) and imaged by fluorescent confocal microscopy (a). The labelled fibronectin was taken up by the cells and incorporated into the fibrous ECM they produced, the overall organization of which is similar to the actin pattern. (b) Intensity profiles averaged over seven lines per corner for three corners (one per pore) were derived from the centre of the pore to the edge of the scaffold for each channel and each time point. The evolution of the position of the peaks of intensity shows that fibronectin is integrated locally in the tissue (scale bar, 100 µm).
Figure 3.
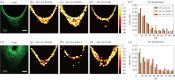
Degree of fibre alignment. The local orientations of actin, Fn546, Fn633 and collagen (SHG) were measured using FFT analysis of the confocal images. Shown in (a) and (f) are confocal images of samples stained for actin at two different time points (days 12 and 28) (scale bar, 100 µm). The degree of fibre alignment is measured by differences in orientation angles (Δ_ϑ_) for actin–Fn546 (b,g), actin–Fn633 (c,h) and actin–collagen (d,i) as well as their associated histograms (e,j). (For histograms of the other experimental images, see electronic supplementary material, figure S4 and table S1.)
Figure 4.
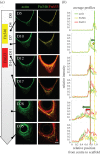
Perturbations of tissue mechanics. Control: ECM-rich tissues grown in the control conditions were fixed after 14 days (a) and 35 days (f) of culture, stained for actin (green) and nuclei (red) and imaged by fluorescent confocal microscopy using SHG signal to visualize collagen fibrils (white). Cell contractility: samples were treated with blebbistatin, either temporarily with 20 µM for 24 h after 14 days of culture (red band, m) or continuously with 2 µM over the whole culture time. In both cases, impairing cell contractility both affected the organization of the tissue (b,c,g,h) and the kinetics of tissue growth (l,m). Growth kinetics of the samples continuously treated with blebbistatin were significantly different to the control (two-way ANOVA, p < 0.001), and significantly different to the control (two-way ANOVA, p < 0.05) for day 7 and from day 18 till day 25 for the pulse treatment of blebbistatin. When treated for 24 h, cells temporarily lost their elongated shape and sharp organization (b,c) and the formation of thick collagen fibres seemed to be impaired after 35 days of growth (h). When treated continuously with a low dose of blebbistatin, global tissue production and organization was impaired compared with the control conditions (g). Extracellular matrix: samples were either treated temporarily with 0.1% collagenase for 2 h after 14 days of culture (red line, n) or continuously deprived with ascorbic acid (ASC) over the whole culture time. In both cases, impairing the stability or formation of collagen prevented the tissue from being tightly anchored to the scaffold as shown by the large holes without cells (d,e). After 35 days of growth, the formation of thick collagen fibres is rare in the treated samples compared with the control condition and a hole in the collagen network suggests that the ECM produced in such conditions cannot bear the tension imposed by the surrounding cells (i,j, arrow). The collagenase treatment suddenly increased the projected tissue area (n), whereas the deprivation of ASC did not significantly (two-way ANOVA) alter tissue growth (o). (Scale bar, 100 µm.) Error bars in (l_–_o) indicate the standard deviation, (n = 9) for each dataset.
Figure 5.
Structural and mechanical transfer from the active cells to the passive ECM. (a) Tissue grown for 35 days with addition of yellow- and red-labelled fibronectin at days 4 and 10, respectively, was fixed, stained for actin (green) and imaged with fluorescent and SHG microscopy to visualize actin, fibronectin and collagen fibres simultaneously. (b) The internal structure of the tissue is well described by a geometrical model based on the sequential assembly of fibrous cellular elements, the organization of which is progressively transferred to fibrous extracellular elements synthetized by the cells. (c) Similarly, we propose a model of mechanical handshake between the cells and their ECM that enables the progressive transfer of the tension actively generated by the contractile actin network into tension born in the passive ECM as the tissue matures (scale bar, 100 µm).
Similar articles
- New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts.
Sivakumar P, Czirok A, Rongish BJ, Divakara VP, Wang YP, Dallas SL. Sivakumar P, et al. J Cell Sci. 2006 Apr 1;119(Pt 7):1350-60. doi: 10.1242/jcs.02830. Epub 2006 Mar 14. J Cell Sci. 2006. PMID: 16537652 - An in vitro assessment of a cell-containing collagenous extracellular matrix-like scaffold for bone tissue engineering.
Pedraza CE, Marelli B, Chicatun F, McKee MD, Nazhat SN. Pedraza CE, et al. Tissue Eng Part A. 2010 Mar;16(3):781-93. doi: 10.1089/ten.TEA.2009.0351. Tissue Eng Part A. 2010. PMID: 19778181 - Nitric oxide regulates cell behavior on an interactive cell-derived extracellular matrix scaffold.
Xing Q, Zhang L, Redman T, Qi S, Zhao F. Xing Q, et al. J Biomed Mater Res A. 2015 Dec;103(12):3807-14. doi: 10.1002/jbm.a.35524. Epub 2015 Jul 1. J Biomed Mater Res A. 2015. PMID: 26074441 Free PMC article. - Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue.
Goh KL, Holmes DF. Goh KL, et al. Int J Mol Sci. 2017 Apr 25;18(5):901. doi: 10.3390/ijms18050901. Int J Mol Sci. 2017. PMID: 28441344 Free PMC article. Review. - Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis.
Kadler K. Kadler K. Birth Defects Res C Embryo Today. 2004 Mar;72(1):1-11. doi: 10.1002/bdrc.20002. Birth Defects Res C Embryo Today. 2004. PMID: 15054900 Review.
Cited by
- Collagen Fibrils Mechanically Contribute to Tissue Contraction in an In Vitro Wound Healing Scenario.
Brauer E, Lippens E, Klein O, Nebrich G, Schreivogel S, Korus G, Duda GN, Petersen A. Brauer E, et al. Adv Sci (Weinh). 2019 Mar 14;6(9):1801780. doi: 10.1002/advs.201801780. eCollection 2019 May 3. Adv Sci (Weinh). 2019. PMID: 31065517 Free PMC article. - Channeling Effect and Tissue Morphology in a Perfusion Bioreactor Imaged by X-Ray Microtomography.
Beauchesne CC, Chabanon M, Smaniotto B, Ladoux B, Goyeau B, David B. Beauchesne CC, et al. Tissue Eng Regen Med. 2020 Jun;17(3):301-311. doi: 10.1007/s13770-020-00246-8. Epub 2020 Apr 20. Tissue Eng Regen Med. 2020. PMID: 32314312 Free PMC article. - Mechanical Cell Interactions on Curved Interfaces.
Buenzli PR, Kuba S, Murphy RJ, Simpson MJ. Buenzli PR, et al. Bull Math Biol. 2025 Jan 7;87(2):29. doi: 10.1007/s11538-024-01406-w. Bull Math Biol. 2025. PMID: 39775998 Free PMC article. - Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts.
Kollmannsberger P, Bidan CM, Dunlop JWC, Fratzl P, Vogel V. Kollmannsberger P, et al. Sci Adv. 2018 Jan 17;4(1):eaao4881. doi: 10.1126/sciadv.aao4881. eCollection 2018 Jan. Sci Adv. 2018. PMID: 29349300 Free PMC article. - Epithelial organ shape is generated by patterned actomyosin contractility and maintained by the extracellular matrix.
Nematbakhsh A, Levis M, Kumar N, Chen W, Zartman JJ, Alber M. Nematbakhsh A, et al. PLoS Comput Biol. 2020 Aug 20;16(8):e1008105. doi: 10.1371/journal.pcbi.1008105. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32817654 Free PMC article.
References
- Weiner S, Wagner HD. 1998. The material bone: structure–mechanical function relations. Annu. Rev. Mater. Sci. 28, 271–298. (10.1146/annurev.matsci.28.1.271) - DOI
- Fratzl P, Weinkamer R. 2007. Nature's hierarchical materials. Prog. Mater. Sci. 52, 1263–1334. (10.1016/j.pmatsci.2007.06.001) - DOI
- Halper J, Kjaer M. 2014. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. In: Progress in heritable soft connective tissue diseases. Advances in experimental medicine and biology (ed. Halper J.). Dordrecht, The Netherlands: Springer. - PubMed
- Fratzl P, editor. 2008. Collagen: structure and mechanics. New York, NY: Springer.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources