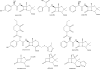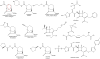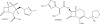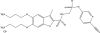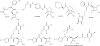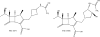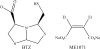New β-Lactamase Inhibitors in the Clinic - PubMed (original) (raw)
Review
New β-Lactamase Inhibitors in the Clinic
Krisztina M Papp-Wallace et al. Infect Dis Clin North Am. 2016 Jun.
Abstract
Given the serious medical burden of β-lactamases, many approaches are being used identify candidate agents for β-lactamase inhibition. Here, we review two β-lactam-β-lactamase inhibitor (BL-BLI) combinations, ceftolozane-tazobactam and ceftazidime-avibactam that recently entered the clinic. In addition, we focus on BL-BLI combinations in preclinical development that have demonstrated activity in clinical isolates via susceptibility testing and/or in in vivo models of infection. We highlight only the BLIs that are able to reduce the Clinical Laboratory Standards Institute (CLSI) breakpoints for the BL partner into the susceptible range. Our analysis includes the primary literature, meeting abstracts, as well as the patent literature.
Keywords: Boronic acids; Carbapenems; Diazabicyclooctanones; Inhibitor; Metallo-beta-lactamases; Monobactams; Sulfones; β-Lactamases.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Fig. 1
β-Lactamase inhibitors of the past and their β-lactam partners.
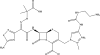
Fig. 2
Chemical structure of ceftolozane.
Fig. 3
DBOs and DBO β-lactam partners.
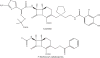
Fig. 4
Chemical structures of the carbavance (meropenem-RPX7009) combination, biapenem, and other boronates.
Fig. 5
Chemical structures of AAI101 and cefepime.
Fig. 6
Phosphonate, MG96077.
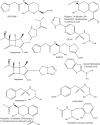
Fig. 7
Chemical structures of monobactams and bridged monobactams.
Fig. 8
Chemical structure of 3′-thiobenzoyl cephalosporins.
Fig. 9
Chemical structures of FSI-1671 and FSI-1686.
Fig. 10
Chemical structure of MBL-specific inhibitors the BTZ, (3R,5R,7aS)-5-(sulfanylmethyl) tetrahydro[1,3] thiazol[4,3-b][1,3]thiazole-3-carboxylic acid and ME1071.
Similar articles
- A resurgence of β-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens.
Bush K. Bush K. Int J Antimicrob Agents. 2015 Nov;46(5):483-93. doi: 10.1016/j.ijantimicag.2015.08.011. Epub 2015 Sep 25. Int J Antimicrob Agents. 2015. PMID: 26498989 Review. - Ceftazidime-Avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms.
Sharma R, Park TE, Moy S. Sharma R, et al. Clin Ther. 2016 Mar;38(3):431-44. doi: 10.1016/j.clinthera.2016.01.018. Epub 2016 Mar 2. Clin Ther. 2016. PMID: 26948862 Review. - The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections.
Papp-Wallace KM. Papp-Wallace KM. Expert Opin Pharmacother. 2019 Dec;20(17):2169-2184. doi: 10.1080/14656566.2019.1660772. Epub 2019 Sep 9. Expert Opin Pharmacother. 2019. PMID: 31500471 Free PMC article. Review. - Focus on ceftazidime-avibactam for optimizing outcomes in complicated intra-abdominal and urinary tract infections.
Nicolau DP. Nicolau DP. Expert Opin Investig Drugs. 2015;24(9):1261-73. doi: 10.1517/13543784.2015.1062873. Epub 2015 Jul 6. Expert Opin Investig Drugs. 2015. PMID: 26145447 Review. - Ceftolozane/tazobactam and ceftazidime/avibactam: two novel β-lactam/β-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections.
Liscio JL, Mahoney MV, Hirsch EB. Liscio JL, et al. Int J Antimicrob Agents. 2015 Sep;46(3):266-71. doi: 10.1016/j.ijantimicag.2015.05.003. Epub 2015 Jun 14. Int J Antimicrob Agents. 2015. PMID: 26143591 Review.
Cited by
- Spectroscopic and biochemical characterization of metallo-β-lactamase IMP-1 with dicarboxylic, sulfonyl, and thiol inhibitors.
Zhang H, Yang K, Cheng Z, Thomas C, Steinbrunner A, Pryor C, Vulcan M, Kemp C, Orea D, Paththamperuma C, Chen AY, Cohen SM, Page RC, Tierney DL, Crowder MW. Zhang H, et al. Bioorg Med Chem. 2021 Jun 15;40:116183. doi: 10.1016/j.bmc.2021.116183. Epub 2021 May 1. Bioorg Med Chem. 2021. PMID: 33965839 Free PMC article. - Editorial: Structural and Biochemical Aspects of the Interaction of β-Lactamases With State-of-the-Art Inhibitors.
Papp-Wallace KM, Docquier JD, Kerff F, Power P. Papp-Wallace KM, et al. Front Microbiol. 2022 Feb 2;13:849324. doi: 10.3389/fmicb.2022.849324. eCollection 2022. Front Microbiol. 2022. PMID: 35185858 Free PMC article. No abstract available. - Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections.
Liu B, Trout REL, Chu GH, McGarry D, Jackson RW, Hamrick JC, Daigle DM, Cusick SM, Pozzi C, De Luca F, Benvenuti M, Mangani S, Docquier JD, Weiss WJ, Pevear DC, Xerri L, Burns CJ. Liu B, et al. J Med Chem. 2020 Mar 26;63(6):2789-2801. doi: 10.1021/acs.jmedchem.9b01518. Epub 2019 Dec 16. J Med Chem. 2020. PMID: 31765155 Free PMC article. - Modulation of the Specificity of Carbapenems and Diazabicyclooctanes for Selective Activity against Mycobacterium tuberculosis.
Barnier JP, Saidjalolov S, Bouchet F, Mayer L, Edoo Z, Sayah I, Iannazzo L, Ethève-Quelquejeu M, Mainardi JL, Braud E, Arthur M. Barnier JP, et al. Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0235721. doi: 10.1128/aac.02357-21. Epub 2022 Aug 9. Antimicrob Agents Chemother. 2022. PMID: 35943263 Free PMC article. - In Vitro and Intracellular Activity of Imipenem Combined with Rifabutin and Avibactam against Mycobacterium abscessus.
Le Run E, Arthur M, Mainardi JL. Le Run E, et al. Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00623-18. doi: 10.1128/AAC.00623-18. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29866869 Free PMC article.
References
- Clinical and Laboratory Standards Institute. Twenty-fifth Informational supplement M100-S25. Wayne (PA): CLSI; 2015. Performance standards for antimicrobial susceptibility testing.
- Takeda S, Ishii Y, Hatano K, et al. Stability of FR264205 against AmpC β-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:443–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI100560/AI/NIAID NIH HHS/United States
- VA999999/Intramural VA/United States
- R01 AI063517/AI/NIAID NIH HHS/United States
- I01 BX001974/BX/BLRD VA/United States
- I01 BX002872/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical