MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism - PubMed (original) (raw)
Review
MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism
Changhan Lee et al. Free Radic Biol Med. 2016 Nov.
Abstract
Mitochondria are ancient organelles that are thought to have emerged from once free-living α-proto-bacteria. As such, they still possess several bacterial-like qualities, including a semi-autonomous genetic system, complete with an independent genome and a unique genetic code. The bacterial-like circular mitochondrial DNA (mtDNA) has been described to encode 37 genes, including 22 tRNAs, 2 rRNAs, and 13 mRNAs. Two additional peptides reported to originate from the mtDNA, namely humanin (Hashimoto et al., 2001; Ikone et al., 2003; Guo et al., 2003) [1-3] and MOTS-c (mitochondrial ORF of the twelve S c) (Lee et al., 2015) [4], indicate a larger mitochondrial genetic repertoire (Shokolenko and Alexeyev, 2015) [5]. These mitochondrial-derived peptides (MDPs) have profound and distinct biological activities and provide a paradigm-shifting concept of active mitochondrial-encoded signals that act at the cellular and organismal level (i.e. mitochondrial hormone) (da Cunha et al., 2015; Quiros et al., 2016) [6,7]. Considering that mitochondria are the single most important metabolic organelle, it is not surprising that these MDPs have metabolic actions. MOTS-c has been shown to target the skeletal muscle and enhance glucose metabolism. As such, MOTS-c has implications in the regulation of obesity, diabetes, exercise, and longevity, representing an entirely novel mitochondrial signaling mechanism to regulate metabolism within and between cells.
Keywords: Aging; Exercise; Fat; Insulin; MOTS-c; Metabolism; Mitochondrial-derived peptides; Muscle.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
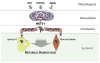
Fig. 1
A model of the systemic metabolic effects of the mitochondrial-derived peptide hormone MOTS-c. Exercise, diet (pro-longevity dietary interventions), and aging may affect the production, secretion, and activity of MOTS-c. Skeletal muscle is a major target of circulating MOTS-c, but experimental evidence also points to fat cells as a potential target. Depending on the environment, MOTS-c may have dual roles as an anabolic and a lipolytic hormone.
Similar articles
- Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease.
Liu C, Gidlund EK, Witasp A, Qureshi AR, Söderberg M, Thorell A, Nader GA, Barany P, Stenvinkel P, von Walden F. Liu C, et al. Am J Physiol Renal Physiol. 2019 Nov 1;317(5):F1122-F1131. doi: 10.1152/ajprenal.00202.2019. Epub 2019 Aug 21. Am J Physiol Renal Physiol. 2019. PMID: 31432706 - Mitochondrial-derived peptides and exercise.
Woodhead JST, Merry TL. Woodhead JST, et al. Biochim Biophys Acta Gen Subj. 2021 Dec;1865(12):130011. doi: 10.1016/j.bbagen.2021.130011. Epub 2021 Sep 11. Biochim Biophys Acta Gen Subj. 2021. PMID: 34520826 Review. - Mitochondrial-derived peptides in energy metabolism.
Merry TL, Chan A, Woodhead JST, Reynolds JC, Kumagai H, Kim SJ, Lee C. Merry TL, et al. Am J Physiol Endocrinol Metab. 2020 Oct 1;319(4):E659-E666. doi: 10.1152/ajpendo.00249.2020. Epub 2020 Aug 10. Am J Physiol Endocrinol Metab. 2020. PMID: 32776825 Free PMC article. Review. - Effects of MOTS-c on the mitochondrial function of cells harboring 3243 A to G mutant mitochondrial DNA.
Ahn CH, Choi EH, Kong BS, Cho YM. Ahn CH, et al. Mol Biol Rep. 2020 May;47(5):4029-4035. doi: 10.1007/s11033-020-05429-z. Epub 2020 Apr 11. Mol Biol Rep. 2020. PMID: 32279209 - The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance.
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. Lee C, et al. Cell Metab. 2015 Mar 3;21(3):443-54. doi: 10.1016/j.cmet.2015.02.009. Cell Metab. 2015. PMID: 25738459 Free PMC article.
Cited by
- Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration.
Nashine S, Cohen P, Nesburn AB, Kuppermann BD, Kenney MC. Nashine S, et al. Sci Rep. 2018 Oct 11;8(1):15175. doi: 10.1038/s41598-018-33290-5. Sci Rep. 2018. PMID: 30310092 Free PMC article. - Multidimensional Biomarker Analysis Including Mitochondrial Stress Indicators for Nonalcoholic Fatty Liver Disease.
Chang E, Chang JS, Kong ID, Baik SK, Kim MY, Park KS. Chang E, et al. Gut Liver. 2022 Mar 15;16(2):171-189. doi: 10.5009/gnl210106. Gut Liver. 2022. PMID: 34420934 Free PMC article. Review. - MOTS-c, the Most Recent Mitochondrial Derived Peptide in Human Aging and Age-Related Diseases.
Mohtashami Z, Singh MK, Salimiaghdam N, Ozgul M, Kenney MC. Mohtashami Z, et al. Int J Mol Sci. 2022 Oct 9;23(19):11991. doi: 10.3390/ijms231911991. Int J Mol Sci. 2022. PMID: 36233287 Free PMC article. Review. - microRNA-222 Attenuates Mitochondrial Dysfunction During Transmissible Gastroenteritis Virus Infection.
Zhao X, Song X, Bai X, Tan Z, Ma X, Guo J, Zhang Z, Du Q, Huang Y, Tong D. Zhao X, et al. Mol Cell Proteomics. 2019 Jan;18(1):51-64. doi: 10.1074/mcp.RA118.000808. Epub 2018 Sep 26. Mol Cell Proteomics. 2019. PMID: 30257878 Free PMC article. - Future Perspectives in Oxidative Stress in Trisomy 13 and 18 Evaluation.
Buczyńska A, Sidorkiewicz I, Hameed A, Krętowski AJ, Zbucka-Krętowska M. Buczyńska A, et al. J Clin Med. 2022 Mar 24;11(7):1787. doi: 10.3390/jcm11071787. J Clin Med. 2022. PMID: 35407395 Free PMC article. Review.
References
- Guo B, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials