CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma - PubMed (original) (raw)
. 2016 Jun 13;29(6):832-845.
doi: 10.1016/j.ccell.2016.04.014. Epub 2016 Jun 2.
Saadia A Karim 1, Joshua D G Leach 1, Peter Bailey 2, Rosanna Upstill-Goddard 2, Loveena Rishi 2, Mona Foth 1, Sheila Bryson 1, Karen McDaid 3, Zena Wilson 3, Catherine Eberlein 3, Juliana B Candido 4, Mairi Clarke 5, Colin Nixon 1, John Connelly 1, Nigel Jamieson 6, C Ross Carter 6, Frances Balkwill 4, David K Chang 2, T R Jeffry Evans 7, Douglas Strathdee 1, Andrew V Biankin 2, Robert J B Nibbs 5, Simon T Barry 3, Owen J Sansom 8, Jennifer P Morton 7
Affiliations
- PMID: 27265504
- PMCID: PMC4912354
- DOI: 10.1016/j.ccell.2016.04.014
CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma
Colin W Steele et al. Cancer Cell. 2016.
Abstract
CXCR2 has been suggested to have both tumor-promoting and tumor-suppressive properties. Here we show that CXCR2 signaling is upregulated in human pancreatic cancer, predominantly in neutrophil/myeloid-derived suppressor cells, but rarely in tumor cells. Genetic ablation or inhibition of CXCR2 abrogated metastasis, but only inhibition slowed tumorigenesis. Depletion of neutrophils/myeloid-derived suppressor cells also suppressed metastasis suggesting a key role for CXCR2 in establishing and maintaining the metastatic niche. Importantly, loss or inhibition of CXCR2 improved T cell entry, and combined inhibition of CXCR2 and PD1 in mice with established disease significantly extended survival. We show that CXCR2 signaling in the myeloid compartment can promote pancreatic tumorigenesis and is required for pancreatic cancer metastasis, making it an excellent therapeutic target.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
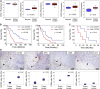
CXCR2 Expression at the Tumor Border Is Associated with Poor Outcome in Human PDAC (A) Expression of CXCR2 and its ligands in the tumor border compared with adjacent normal pancreas within pancreaticoduodenectomy specimens (n = 44). RNA was prepared from whole targeted biopsies of the edges of tumors, post-resection, and from adjacent normal pancreas. p Values, Mann-Whitney U test. (B) Kaplan-Meier analysis of survival in terms of low or high CXCR2 and CXCL2 expression from RNA from whole targeted biopsies of the edges of resected tumors. p Values, log rank test. (C) Kaplan-Meier analysis of survival in terms of low or high CXCR2 expression as assessed by IHC on full-face sections of tumor border regions (n = 11). p Values, log rank test. (D) IHC staining for CXCR2, MPO, CD68, and CD3 in the stroma at the edge of resected tumors. Arrowheads indicate direction of invasion into either adjacent duodenum or normal pancreas. Scale bars represent 500 μm. Boxplots below show quantification of cells staining positive for each marker in the tumor center versus tumor border (n ≥ 3). p Values, Mann-Whitney U test. The region 1 mm proximal to the adjacent normal tissue was assessed in (C and D). See also Figure S1.
Figure 2
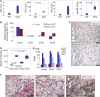
KPC Mice Recapitulate the Microenvironment and CXCR2 Expression of Human PDAC (A) Expression of Cxcr2 and its ligands in KPC PDAC (n = 6) compared with normal WT pancreas. p Values, Mann-Whitney U test. (B) Expression of Cxcl1, Cxcl2, Cxcl5, and Cxcr2 from pooled (n = 3) laser-capture micro-dissected stroma or tumor epithelium compared with WT pancreas. Expression normalized to Gapdh. p Value, ANOVA. Error bars are ±SEM. (C) Representative IHC for CXCL2 and CXCR2 in PDAC from KPC mice. Scale bars represent 200 μm. (D) Cytokine array analysis of CXCR2 ligands produced by KPC cell lines compared with control pancreatic duct epithelial cells (n = 6). p Values, Mann-Whitney U test. (E) RNA-seq expression of Cxc1, Cxcl2, Cxcl5, Cxcr2, and Mpo in FAP+ fibroblasts from normal pancreas, PanIN, or PDAC from KPC mice (n = 2). Error bars are ±SD. (F) Dual IHC for CXCL1 (red) and CK19, α-SMA, or MPO (brown) in KPC tumors. See also Figure S2.
Figure 3
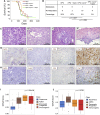
Cxcr2 Deletion Inhibits Metastasis in KPC Mice (A) Kaplan-Meier survival analysis of KPC and KPC Cxcr2 −/− mice untreated, or treated from 10 weeks of age with 100 mg/kg gemcitabine, n = 21, 24, 10, 13, respectively (not significant, log rank test). (B) Table comparing incidence of metastases in KPC and KPC Cxcr2 −/− mice treated as indicated. p Values, chi-square test. (C and D) H&E staining of representative primary tumors from (C) KPC and (D) KPC Cxcr2 −/− mice. (E and F) H&E staining of representative (E) liver and (F) diaphragm metastasis from KPC mice. (G and H) IHC for MPO, F4/80, CD3, and tenascin C (TNC) in tumors from (G) KPC and (H) KPC Cxcr2 −/− mice. (I and J) Boxplots of signature scores (I) upregulated and (J) downregulated, in KPC Cxcr2 −/− mice, stratified by human PDAC class. p Values, Kruskall-Wallis test. See also Figure S3.
Figure 4
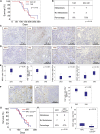
Neutrophil Ablation Also Inhibits Metastasis in the KPC Model (A) Kaplan-Meier analysis of survival of KPC mice treated from 10 weeks of age with 2A3 isotype-control antibody (n = 11) or 1A8, anti-Ly6G neutrophil-ablating antibody (n = 15). p Values, log rank test. (B) Table comparing incidence of metastases in KPC mice treated with 2A3 or 1A8. p Value, chi-square test. (C and D) IHC on tumors from (C) 2A3- or (D) 1A8-treated mice, for MPO, 1A8, F480, and CD3. (E) Boxplots showing quantification of IHC in (C) and (D). p Values, Mann-Whitney U test. (F) IHC for 1A8 on spleens from 2A3- and 1A8-treated mice, quantified on right. p Values, Mann-Whitney U test. (G) Kaplan-Meier survival analysis of KPC (n = 19, median = 157 days) and KPC Cxcr2 fl/fl mice (n = 28, median = 141 days). p Values, log rank test. (H) Table comparing incidence of metastases in KPC and KPC Cxcr2 fl/fl mice. p Values, chi-square test. (I) Boxplots showing quantification of CD3 IHC in tumors from KPC and KPC Cxcr2 fl/fl mice. p Value, Mann-Whitney U test.
Figure 5
Cxcr2 Inhibition Inhibits Metastasis and Prolongs Survival in KPC Mice (A) Kaplan-Meier analysis of KPC mice treated from 10 weeks of age with scrambled pepducin (n = 15), gemcitabine (n = 14), CXCR2-inhibiting pepducin (n = 20), or CXCR2-inhibiting pepducin + gemcitabine (n = 11). p Values, log rank test. (B) Table comparing incidence of metastases in KPC mice treated as indicated. p Values, chi-square test. (C and D) H&E staining and IHC for MPO, F4/80, CD3, and tenascin C (TNC) in tumors in response to (C) scrambled pepducin and (D) CXCR2-targeting pepducin. See also Figure S4.
Figure 6
Therapeutic Targeting of CXCR2 Inhibits Metastasis and Prolongs Survival in KPC Mice (A) Boxplot showing circulating neutrophils in CXCR2 SM-treated mice (n = 4). p Values, Mann-Whitney U test. (B) Kaplan-Meier survival analysis of KPC mice treated from 10 weeks of age with vehicle (n = 11), gemcitabine (n = 14), CXCR2 SM (n = 15), or CXCR2 SM + gemcitabine (n = 12). p Values, log rank test. (C) Table comparing incidence of metastases in KPC mice treated as indicated. p Values, chi-square test. (D–F) H&E staining and IHC for MPO, F4/80, CD3, and tenascin C (TNC) in tumors in response to (D) vehicle, (E) CXCR2 SM, and (F) CXCR2 SM + gemcitabine. See also Figure S5 and Table S1.
Figure 7
CXCR2 Blockade Promotes T Cell Infiltration into Tumors and Sensitivity to Immunotherapy (A) Kaplan-Meier survival analysis of tumor-bearing KPC mice treated with either gemcitabine, CXCR2 SM alone for 2 weeks, and then in combination with anti-PD1, vehicle alone for 2 weeks, and then combined with anti-PD1, CXCR2 SM alone (censors on pink line), or vehicle alone (censors on cyan line). Few mice on vehicle alone survived for 2 weeks to allow PD1 treatment as shown in the table below. p Values, chi-square test. (B and C) IHC for Ki67 in tumors from KPC mice treated with (B) vehicle + PD1 or (C) CXCR2 SM + PD1. (D and E) IHC for cleaved caspase 3 in tumors from KPC mice treated with (D) vehicle + PD1 or (E) CXCR2 SM + PD1. (F) FACS analysis of intratumoral CD3+ cells in mice treated as indicated. (G) Boxplot showing quantification of IHC for CD4+ and CD8+ T cells in tumors from KPC mice treated as indicated. (H) FACS analysis of intratumoral CD4+, CD8+, CD4+CD25+, and NK1.1+ cells (% of CD3+ cells) in mice treated as indicated. (I) FACS profile of CD4+ and CD8+ T cells isolated from tumors in mice treated with either vehicle + anti-PD1 or CXCR2 SM + anti-PD1. (F–I) n = 3. p Values, Mann-Whitney U test.
Comment in
- Pancreatic Cancer: Planning Ahead for Metastatic Spread.
Stromnes IM, Greenberg PD. Stromnes IM, et al. Cancer Cell. 2016 Jun 13;29(6):774-776. doi: 10.1016/j.ccell.2016.05.013. Cancer Cell. 2016. PMID: 27300430
Similar articles
- Targeting ESE3/EHF With Nifurtimox Inhibits CXCR2+ Neutrophil Infiltration and Overcomes Pancreatic Cancer Resistance to Chemotherapy and Immunotherapy.
Xie Y, Zhou T, Li X, Zhao K, Bai W, Hou X, Liu Z, Ni B, Zhang Z, Yan J, Wang Y, Jiang W, Wang H, Chang A, Gao S, Zhao T, Yang S, Huang C, Liu J, Hao J. Xie Y, et al. Gastroenterology. 2024 Jul;167(2):281-297. doi: 10.1053/j.gastro.2024.02.046. Epub 2024 Mar 15. Gastroenterology. 2024. PMID: 38492894 - A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model.
Rajeshkumar NV, Rasheed ZA, García-García E, López-Ríos F, Fujiwara K, Matsui WH, Hidalgo M. Rajeshkumar NV, et al. Mol Cancer Ther. 2010 Sep;9(9):2582-92. doi: 10.1158/1535-7163.MCT-10-0370. Epub 2010 Jul 26. Mol Cancer Ther. 2010. PMID: 20660600 Free PMC article. - CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma.
Chao T, Furth EE, Vonderheide RH. Chao T, et al. Cancer Immunol Res. 2016 Nov;4(11):968-982. doi: 10.1158/2326-6066.CIR-16-0188. Epub 2016 Oct 13. Cancer Immunol Res. 2016. PMID: 27737879 Free PMC article. - Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma.
Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, Linehan DC. Nywening TM, et al. Gut. 2018 Jun;67(6):1112-1123. doi: 10.1136/gutjnl-2017-313738. Epub 2017 Dec 1. Gut. 2018. PMID: 29196437 Free PMC article. - Gemcitabine resistance in pancreatic ductal adenocarcinoma.
Binenbaum Y, Na'ara S, Gil Z. Binenbaum Y, et al. Drug Resist Updat. 2015 Nov;23:55-68. doi: 10.1016/j.drup.2015.10.002. Epub 2015 Nov 3. Drug Resist Updat. 2015. PMID: 26690340 Review.
Cited by
- Convergent inducers and effectors of T cell paralysis in the tumour microenvironment.
Hanahan D, Michielin O, Pittet MJ. Hanahan D, et al. Nat Rev Cancer. 2024 Oct 24. doi: 10.1038/s41568-024-00761-z. Online ahead of print. Nat Rev Cancer. 2024. PMID: 39448877 Review. - The Role of Tumor Microenvironment in Pancreatic Cancer Immunotherapy: Current Status and Future Perspectives.
Poyia F, Neophytou CM, Christodoulou MI, Papageorgis P. Poyia F, et al. Int J Mol Sci. 2024 Sep 3;25(17):9555. doi: 10.3390/ijms25179555. Int J Mol Sci. 2024. PMID: 39273502 Free PMC article. Review. - A key regulator of tumor-associated neutrophils: the CXCR2 chemokine receptor.
Kang W, Wang C, Wang M, Liu M, Hu W, Liang X, Yang J, Zhang Y. Kang W, et al. J Mol Histol. 2024 Dec;55(6):1051-1061. doi: 10.1007/s10735-024-10260-y. Epub 2024 Sep 13. J Mol Histol. 2024. PMID: 39269537 Review. - Barriers and opportunities in pancreatic cancer immunotherapy.
Ju Y, Xu D, Liao MM, Sun Y, Bao WD, Yao F, Ma L. Ju Y, et al. NPJ Precis Oncol. 2024 Sep 12;8(1):199. doi: 10.1038/s41698-024-00681-z. NPJ Precis Oncol. 2024. PMID: 39266715 Free PMC article. Review. - Immune status and combined immunotherapy progression in Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant tumors.
He D, Bai R, Chen N, Cui J. He D, et al. Chin J Cancer Res. 2024 Aug 30;36(4):421-441. doi: 10.21147/j.issn.1000-9604.2024.04.06. Chin J Cancer Res. 2024. PMID: 39246706 Free PMC article.
References
- Acosta J.C., O'Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. - PubMed
- Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C., Miller D.K., Christ A.N., Bruxner T.J., Quinn M.C. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. - PubMed
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0900992/MRC_/Medical Research Council/United Kingdom
- 20265/CRUK_/Cancer Research UK/United Kingdom
- 21139/CRUK_/Cancer Research UK/United Kingdom
- 12946/CRUK_/Cancer Research UK/United Kingdom
- 16354/CRUK_/Cancer Research UK/United Kingdom
- FLF2015_04_GLASGOW/PANCREATICCANUK_/Pancreatic Cancer UK/United Kingdom
- 20409/CRUK_/Cancer Research UK/United Kingdom
- 11650/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 17263/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases