Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus - PubMed (original) (raw)
Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus
Anurodh Shankar Agrawal et al. Hum Vaccin Immunother. 2016 Sep.
Abstract
To determine if a hypersensitive-type lung pathology might occur when mice were given an inactivated MERS-CoV vaccine and challenged with infectious virus as was seen with SARS-CoV vaccines, we prepared and vaccinated mice with an inactivated MERS-CoV vaccine. Neutralizing antibody was induced by vaccine with and without adjuvant and lung virus was reduced in vaccinated mice after challenge. Lung mononuclear infiltrates occurred in all groups after virus challenge but with increased infiltrates that contained eosinophils and increases in the eosinophil promoting IL-5 and IL-13 cytokines only in the vaccine groups. Inactivated MERS-CoV vaccine appears to carry a hypersensitive-type lung pathology risk from MERS-CoV infection that is similar to that found with inactivated SARS-CoV vaccines from SARS-CoV infection.
Keywords: coronavirus; Eosinophils; immunopathology; Middle East Respiratory Syndrome; vaccination.
Figures
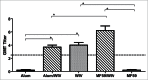
Figure 1.
Mean serum-neutralizing antibody titers to MERS-CoV of vaccinated mice 3 weeks after the second immunization. Alum and MF59 are adjuvant only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum adjuvant, MF59/WIV is WIV formulated with MF59 adjuvant. The serum neutralizing antibody titers are expressed as Geometric Mean Titer (GMT) based on a 2-fold dilution sequence beginning at 1:2 (Log2). * Significantly different (P < 0.01) after correcting for multiple comparisons.
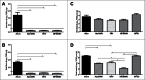
Figure 2.
Mean viral titers of MERS-CoV on days 3 and 6 after intranasal challenge of vaccinated mice with 100 LD50 of MERS-CoV. Lung homogenates and total RNAs extracted from tissues of vaccinated mice at days 3 and 6 post challenge with MERS-CoV were subjected to Vero E6 cell-based infectivity assay and one-step real-time RT-PCR analyses targeting the upE gene of MERS-CoV for assessing viral loads, as previously described (5,6). A serial 10-fold diluted MERS-CoV stock with a titer of 107 TCID50/ml was included in parallel during the quantitative PCR assays to calculate and express the levels of upE gene expression in individual specimens as log10 TCID50 equivalents per gram of tissue. Alum and MF59 are adjuvant-only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum, MF59/WIV is WIV formulated with MF59. A: Vero E6-based infectious viral titers at Day 3, B: Vero E6-based infectious viral titers at Day 6, C: RT-PCR-based viral load at Day 3, and D: RT-PCR-based viral load at Day 6. * Significantly different (P < 0.01) after correcting for multiple comparisons.
Figure 3.
Representative photomicrographs of lung tissue 3 days after challenge of previously vaccinated mice with MERS-CoV. Lung sections were stained with an antibody directed specifically against eosinophilic major basic protein as described (3); eosinophils are brown. The vaccine groups (alum only, MF59 only, WIV only, WIV plus Alum and WIV plus MF59) and the eosinophil infiltration severity score (E0 and E2) are noted on the micrograph; E0 is none, E2 is moderate.
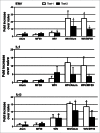
Figure 4.
Mean lung cytokine levels on day 3 after challenge of vaccinated mice with MERS-CoV. Alum and MF59 are adjuvant only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum, MF59/WIV is WIV formulated with MF59. Test 1 and test 2 are separate day tests of the same lung tissue specimen. Results are mean fold increase over naïve transgenic mice based on ΔCt values of each group in reference to those of the internal mouse GAPDH gene. * Significantly greater than for the naïve mouse group (P < 0.01) after correcting for multiple comparisons; ** P = 0.026.
Similar articles
- Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus.
Deng Y, Lan J, Bao L, Huang B, Ye F, Chen Y, Yao Y, Wang W, Qin C, Tan W. Deng Y, et al. Emerg Microbes Infect. 2018 Apr 4;7(1):60. doi: 10.1038/s41426-018-0056-7. Emerg Microbes Infect. 2018. PMID: 29618723 Free PMC article. - A Highly Immunogenic, Protective, and Safe Adenovirus-Based Vaccine Expressing Middle East Respiratory Syndrome Coronavirus S1-CD40L Fusion Protein in a Transgenic Human Dipeptidyl Peptidase 4 Mouse Model.
Hashem AM, Algaissi A, Agrawal AS, Al-Amri SS, Alhabbab RY, Sohrab SS, S Almasoud A, Alharbi NK, Peng BH, Russell M, Li X, Tseng CK. Hashem AM, et al. J Infect Dis. 2019 Oct 8;220(10):1558-1567. doi: 10.1093/infdis/jiz137. J Infect Dis. 2019. PMID: 30911758 Free PMC article. - The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection.
Jiaming L, Yanfeng Y, Yao D, Yawei H, Linlin B, Baoying H, Jinghua Y, Gao GF, Chuan Q, Wenjie T. Jiaming L, et al. Vaccine. 2017 Jan 3;35(1):10-18. doi: 10.1016/j.vaccine.2016.11.064. Epub 2016 Nov 26. Vaccine. 2017. PMID: 27899228 Free PMC article. - Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review.
Choi J, Kim MG, Oh YK, Kim YB. Choi J, et al. Expert Opin Ther Pat. 2017 Jun;27(6):721-731. doi: 10.1080/13543776.2017.1281248. Epub 2017 Jan 25. Expert Opin Ther Pat. 2017. PMID: 28121202 Review. - Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development.
Mubarak A, Alturaiki W, Hemida MG. Mubarak A, et al. J Immunol Res. 2019 Apr 7;2019:6491738. doi: 10.1155/2019/6491738. eCollection 2019. J Immunol Res. 2019. PMID: 31089478 Free PMC article. Review.
Cited by
- Soluble Spike DNA Vaccine Provides Long-Term Protective Immunity against SARS-CoV-2 in Mice and Nonhuman Primates.
Seo YB, Suh YS, Ryu JI, Jang H, Oh H, Koo BS, Seo SH, Hong JJ, Song M, Kim SJ, Sung YC. Seo YB, et al. Vaccines (Basel). 2021 Mar 24;9(4):307. doi: 10.3390/vaccines9040307. Vaccines (Basel). 2021. PMID: 33804981 Free PMC article. - Perspectives on development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Zhang C, Zhou C, Shi L, Liu G. Zhang C, et al. Hum Vaccin Immunother. 2020 Oct 2;16(10):2366-2369. doi: 10.1080/21645515.2020.1787064. Epub 2020 Sep 22. Hum Vaccin Immunother. 2020. PMID: 32961082 Free PMC article. Review. - Vaccine Candidate Against COVID-19 Based on Structurally Modified Plant Virus as an Adjuvant.
Kovalenko AO, Ryabchevskaya EM, Evtushenko EA, Manukhova TI, Kondakova OA, Ivanov PA, Arkhipenko MV, Gushchin VA, Nikitin NA, Karpova OV. Kovalenko AO, et al. Front Microbiol. 2022 Feb 28;13:845316. doi: 10.3389/fmicb.2022.845316. eCollection 2022. Front Microbiol. 2022. PMID: 35295298 Free PMC article. - Nano alum: A new solution to the new challenge.
Lu Y, Liu G. Lu Y, et al. Hum Vaccin Immunother. 2022 Nov 30;18(5):2060667. doi: 10.1080/21645515.2022.2060667. Epub 2022 Apr 26. Hum Vaccin Immunother. 2022. PMID: 35471916 Free PMC article. Review. - An Established Th2-Oriented Response to an Alum-Adjuvanted SARS-CoV-2 Subunit Vaccine Is Not Reversible by Sequential Immunization with Nucleic Acid-Adjuvanted Th1-Oriented Subunit Vaccines.
Cao H, Yang S, Wang Y, Luan N, Yin X, Lin K, Liu C. Cao H, et al. Vaccines (Basel). 2021 Nov 1;9(11):1261. doi: 10.3390/vaccines9111261. Vaccines (Basel). 2021. PMID: 34835192 Free PMC article.
References
- Severe acute respiratory syndrome (SARS) Status of the outbreak and lessons for the immediate future; unmasking a new disease. http://www.who.int/csr/media/sars_wha.pdf
- Middle East respiratory syndrome coronavirus (MERS-CoV) Summary and risk assessment of the current situation in Korea and China – as of 3 June 2015. http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-3j...
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS One 2012; 7:e35421; PMID:22536382; http://dx.doi.org/10.1371/journal.pone.0035421 - DOI - PMC - PubMed
- Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, Baric RS. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 2011; 85:12201-15; PMID:21937658; http://dx.doi.org/10.1128/JVI.06048-11 - DOI - PMC - PubMed
- Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015; 89:3659-70; PMID:25589660; http://dx.doi.org/10.1128/JVI.03427-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous