An E3-ligase-based method for ablating inhibitory synapses - PubMed (original) (raw)
doi: 10.1038/nmeth.3894. Epub 2016 Jun 6.
Christoph Straub 2, Jimena Perez-Sanchez 3 4, William P Dempsey 1, Jason A Junge 1, Richard W Roberts 5, Le A Trinh 1, Scott E Fraser 1, Yves De Koninck 3 4, Paul De Koninck 3 4, Bernardo L Sabatini 2, Don B Arnold 1
Affiliations
- PMID: 27271196
- PMCID: PMC5312699
- DOI: 10.1038/nmeth.3894
An E3-ligase-based method for ablating inhibitory synapses
Garrett G Gross et al. Nat Methods. 2016 Aug.
Abstract
Although neuronal activity can be modulated using a variety of techniques, there are currently few methods for controlling neuronal connectivity. We introduce a tool (GFE3) that mediates the fast, specific and reversible elimination of inhibitory synaptic inputs onto genetically determined neurons. GFE3 is a fusion between an E3 ligase, which mediates the ubiquitination and rapid degradation of proteins, and a recombinant, antibody-like protein (FingR) that binds to gephyrin. Expression of GFE3 leads to a strong and specific reduction of gephyrin in culture or in vivo and to a substantial decrease in phasic inhibition onto cells that express GFE3. By temporarily expressing GFE3 we showed that inhibitory synapses regrow following ablation. Thus, we have created a simple, reversible method for modulating inhibitory synaptic input onto genetically determined cells.
Figures
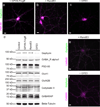
Figure 1. GFE3 specifically ablates Gephyrin
(a–c) Immunostaining showing Gephyrin (green) and PSD-95 (magenta) localization at inhibitory and excitatory synapses, respectively, after expression of GPHN.FingR (a), RandE3 (b) or GFE3 (c) in cultured rat cortical neurons. (d) Western blot showing the expression of Gephyrin, the alpha1 subunit of the GABAA receptor, PSD-95, the GluA1 subunit of the AMPA receptor, the GluN2B subunit of the NMDA receptor, or the Gephyrin interacting proteins Collybistin II and GABARAP in neurons not transduced (Control) or transduced with GFE3, RandE3 or GPHN.FingR. Gephyrin expression is reduced in neurons expressing GFE3 (80 ± 3%, P = 0.01, Kruskal-Wallis). No significant change is observed in the steady-state levels of the other proteins (P > 0.8, Kruskal-Wallis; n = 4 replicate blots). (e, f) Staining for the alpha1 subunit of the GABAA receptor (magenta) and Gephyrin (green) in cortical neuron expressing RandE3 (e) or GFE3 (f). Scale bar represents 10 µm.
Figure 2. Gephyrin ablation reduces the amplitude and frequency of mIPSCs without affecting GABA-evoked currents
(a) Representative traces of mIPSCs obtained from dissociated rat hippocampal neurons expressing GFE3 or RandE3, or control neurons. Cells expressing GFE3 exhibit a significant reduction in frequency (b; P < 0.002, Kruskal-Wallis) and amplitude (**c**; _P_ < 0.02, Kruskal-Wallis) of miniature inhibitory postsynaptic currents (mIPSCs) vs. cells expressing RandE3 or control cells. (**d**) Histogram of the distribution of mIPSC amplitude in a control cell and in a cell expressing GFE3. n = 5 cells for control, n = 6 cells for GFE3 and RandE3. (**e**). Quantification of the peak amplitude of evoked GABA currents recorded in GFE3 transfected and control cells (**f**; _P_ > 0. 5, Mann-Whitney). n = 7 independent experiments. Bars indicate means ± SEM.
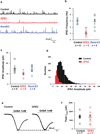
Figure 3. Loss of synaptic GABAergic currents in the intact mouse brain from expression of GFE3
(a) Schematic of experimental layout. (b) Evoked synaptic currents in control and GFE3-expressing indirect pathway spiny projection neurons (iSPNs) in control conditions (black) and after slices were exposed to SR95331 (10 µM), a GABAA receptor antagonist (red). (c) Spontaneous inhibitory currents (sIPSCs, top) were reduced by GFE3 compared with control in both frequency (P < 0.0008, Mann-Whitney) and amplitude (_P_ < 0.03, Mann-Whitney), but no effect was observed on the frequency (_P_ > 0.9, Mann-Whitney) or amplitude (P > 0.17, Mann-Whitney) of excitatory currents (sEPSCs, bottom). Representative example traces are shown on the left, quantification summaries on the right. (d) Tonic GABAA receptor current was quantified as the change in baseline holding current following application of SR95331 (10 µM). (e) Expression of GFE3 resulted in a tendency to reduction of tonic GABAA receptor current, (P = 0.12, Mann-Whitney). For all experiments, grey circles indicate individual cells from a total of 4 animals (inhibitory inputs) or 2 animals (excitatory inputs), bars indicate means ± SEM.
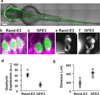
Figure 4. GFE3 expression in zebrafish spinal cord results in Gephyrin ablation and behavioral deficits
(a) A confocal image of a live zebrafish age 2 days post fertilization (dpf) shows mosaic expression of GFE3-GFP driven by an enhancer element of the mnx1 gene that restricts expression to spinal motoneurons. (b,c) Immunostaining of Gephyrin (magenta) and GFP (green) in motoneurons expressing RandE3-GFP (b) or GFE3 (c). Scale bar represents 5 µm. (d) Gephyrin levels in cells expressing RandE3-GFP (64 ± 5 a.u.; n = 5 fish, 6 cells per fish, 3 independent experiments) and in cells expressing GFE3-GFP (23 ± 2 a.u.; P < 0.008, Mann-Whitney; n = 5 fish, 6 cells per fish, 3 independent experiments). (**e**) Zebrafish expressing RandE3-GFP, age 24 hours post fertilization, exhibit normal spontaneous tail flicking, including touching tip of the tail to the base. (**f**) Zebrafish expressing GFE3-GFP in motoneurons are unable to curl their tails sufficiently to touch the tip to the base. (**g**) Quantitation of the minimum distance between the base of the tail and the tip, measured during 5 spontaneous flicks, reveals a > 100% increase in zebrafish that express GFE3-GFP vs. those expressing RandE3-GFP (P < 0.003, Mann-Whitney; n = 5 fish, 5 flicks per fish). Bars indicate means ± SEM.
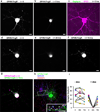
Figure 5. GFE3 mediates transient and reversible ablation of Gephyrin
(a,b) GPHN.FingR-GFP localization in a rat cortical neuron co-expressing GPHN.FingR-GFP and doxycycline(dox)-inducible GFE3-TagRFP before (a) and after (b) addition of dox (1 µg/ml) for 5 hours. (c) Immunostaining of GFE3-TagRFP (red) and Gephyrin (green) in the same cell as in (a) and (b). (d) GPHN.FingR-GFP localization in a rat cortical neuron co-expressing GPHN.FingR-GFP and dox-inducible GFE3-TagRFP before (d) and after addition of dox (1 µg/ml) for 24 hours (e) as well as after subsequent removal of dox for 48 hours (f). (g) An overlay of time points t = 0 (d) and t = 72 hrs (f). (h) Immunostaining of the neuron in (f) for GPHN.FingR-GFP (green), Gephyrin (red), and GAD-65 (blue) at t = 72 hrs. Inset: arrowheads point to puncta where all three proteins are colocalized. Scale bars represent 10 µm. (i) Quantitation of relative synaptic strength, by measuring total intensity of puncta labeled with GPHN.FingR-GFP, indicates that no significant difference exists between synapses at 0 hrs and at 72 hrs in neurons treated with dox or with vehicle (P > 0.2, Mann-Whitney; n = 7 cells, 2 independent experiments).
Similar articles
- Modulation of hippocampal synapse maturation by activity-regulated E3 ligase via non-canonical pathway.
Kumari P, Srinivasan B, Banerjee S. Kumari P, et al. Neuroscience. 2017 Nov 19;364:226-241. doi: 10.1016/j.neuroscience.2017.08.057. Epub 2017 Sep 8. Neuroscience. 2017. PMID: 28890050 - The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin.
Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. Mukherjee J, et al. J Neurosci. 2011 Oct 12;31(41):14677-87. doi: 10.1523/JNEUROSCI.2001-11.2011. J Neurosci. 2011. PMID: 21994384 Free PMC article. - E3 ubiquitin ligases LNX1 and LNX2 localize at neuronal gap junctions formed by connexin36 in rodent brain and molecularly interact with connexin36.
Lynn BD, Li X, Hormuzdi SG, Griffiths EK, McGlade CJ, Nagy JI. Lynn BD, et al. Eur J Neurosci. 2018 Nov;48(9):3062-3081. doi: 10.1111/ejn.14198. Epub 2018 Nov 2. Eur J Neurosci. 2018. PMID: 30295974 - Gephyrin: a master regulator of neuronal function?
Tyagarajan SK, Fritschy JM. Tyagarajan SK, et al. Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review. - Spatial organization of ubiquitin ligase pathways orchestrates neuronal connectivity.
Yamada T, Yang Y, Bonni A. Yamada T, et al. Trends Neurosci. 2013 Apr;36(4):218-26. doi: 10.1016/j.tins.2012.12.004. Epub 2013 Jan 17. Trends Neurosci. 2013. PMID: 23332798 Free PMC article. Review.
Cited by
- Brainstem control of vocalization and its coordination with respiration.
Park J, Choi S, Takatoh J, Zhao S, Harrahill A, Han BX, Wang F. Park J, et al. Science. 2024 Mar 8;383(6687):eadi8081. doi: 10.1126/science.adi8081. Epub 2024 Mar 8. Science. 2024. PMID: 38452069 Free PMC article. - Falling apart.
Marvin JS, Looger LL. Marvin JS, et al. Elife. 2016 Jun 27;5:e18203. doi: 10.7554/eLife.18203. Elife. 2016. PMID: 27345573 Free PMC article. - GABA type a receptor trafficking and the architecture of synaptic inhibition.
Lorenz-Guertin JM, Jacob TC. Lorenz-Guertin JM, et al. Dev Neurobiol. 2018 Mar;78(3):238-270. doi: 10.1002/dneu.22536. Epub 2017 Sep 19. Dev Neurobiol. 2018. PMID: 28901728 Free PMC article. Review. - Relocation of an Extrasynaptic GABAA Receptor to Inhibitory Synapses Freezes Excitatory Synaptic Strength and Preserves Memory.
Davenport CM, Rajappa R, Katchan L, Taylor CR, Tsai MC, Smith CM, de Jong JW, Arnold DB, Lammel S, Kramer RH. Davenport CM, et al. Neuron. 2021 Jan 6;109(1):123-134.e4. doi: 10.1016/j.neuron.2020.09.037. Epub 2020 Oct 22. Neuron. 2021. PMID: 33096025 Free PMC article. - Tuning GABAergic Inhibition: Gephyrin Molecular Organization and Functions.
Pizzarelli R, Griguoli M, Zacchi P, Petrini EM, Barberis A, Cattaneo A, Cherubini E. Pizzarelli R, et al. Neuroscience. 2020 Jul 15;439:125-136. doi: 10.1016/j.neuroscience.2019.07.036. Epub 2019 Jul 26. Neuroscience. 2020. PMID: 31356900 Free PMC article. Review.
References
- Cabot JB, Bushnell A, Alessi V, Mendell NR. Postsynaptic gephyrin immunoreactivity exhibits a nearly one-to-one correspondence with gamma-aminobutyric acid-like immunogold-labeled synaptic inputs to sympathetic preganglionic neurons. J Comp Neurol. 1995;356:418–432. - PubMed
- Feng G, et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. - PubMed
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. - PubMed
- McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nature reviews. Genetics. 2002;3:737–747. - PubMed
Methods-only References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI085583/AI/NIAID NIH HHS/United States
- R37 NS046579/NS/NINDS NIH HHS/United States
- R01 NS081678/NS/NINDS NIH HHS/United States
- R01 GM083898/GM/NIGMS NIH HHS/United States
- R01 NS081687/NS/NINDS NIH HHS/United States
- R01 NS046579/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous