Principles and Properties of Stress Granules - PubMed (original) (raw)
Review
Principles and Properties of Stress Granules
David S W Protter et al. Trends Cell Biol. 2016 Sep.
Abstract
Stress granules are assemblies of untranslating messenger ribonucleoproteins (mRNPs) that form from mRNAs stalled in translation initiation. Stress granules form through interactions between mRNA-binding proteins that link together populations of mRNPs. Interactions promoting stress granule formation include conventional protein-protein interactions as well as interactions involving intrinsically disordered regions (IDRs) of proteins. Assembly and disassembly of stress granules are modulated by various post-translational modifications as well as numerous ATP-dependent RNP or protein remodeling complexes, illustrating that stress granules represent an active liquid wherein energy input maintains their dynamic state. Stress granule formation modulates the stress response, viral infection, and signaling pathways. Persistent or aberrant stress granule formation contributes to neurodegenerative disease and some cancers.
Keywords: RNP granule; intrinsically disordered protein; phase separation; stress granule.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
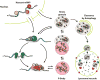
Figure 1. Stress Granules are dynamic and have multiple fates
Stress granules form from untranslating mRNPs. They can interact with P-bodies, exchange components with the cytoplasm, and undergo autophagy.
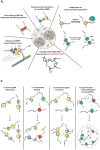
Figure 2. Intermolecular interactions that drive stress granule assembly
A) A diverse set of intermolecular interactions are important for granule assembly. B) Various mechanisms by which intrinsically disordered regions could contribute to granule assembly.
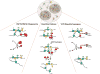
Figure 3. Two models for discrete phases of stress granule assembly
Stress granules can by hypothesized to undergo three phases of assembly. A) In the “Cores First” model, cores precede assembly of large stress granules. The first phase of assembly is the nucleation of translationally repressed RNPs into oligomers. The second phase of assembly is growth of these oligomers into larger assemblies by addition of more translationally repressed RNPs. The third phase of assembly is fusion of these core assemblies and recruitment of the dynamic shell to form the large, microscopically visible granules typically observed in cells. Some of the stability of cores may be due to amyloid interactions, as indicated by squiggly red lines. B) In the “LLPS First” model, the formation of large stress granules precedes core assembly. The first phase of this model is the nucleation of translationally repressed RNPs into initial phase separated droplets, held together by weak dynamics interactions. The second phase is growth of initial droplets by the addition of translationally repressed RNPs. The third phase of assembly is core formation within phase separated granules due to the high local concentration of proteins within the droplets. In this model, the formation of cores may be driven in part by amyloid interactions, as indicated by squiggly red lines.
Figure 4. Various ATPases impact granule assembly
Heat shock proteins, helicases, and VCP all impact stress granule assembly by remodeling specific interactions utilizing the energy of ATP hydrolysis.
Similar articles
- Distinct stages in stress granule assembly and disassembly.
Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R. Wheeler JR, et al. Elife. 2016 Sep 7;5:e18413. doi: 10.7554/eLife.18413. Elife. 2016. PMID: 27602576 Free PMC article. - Multiple Modes of Protein-Protein Interactions Promote RNP Granule Assembly.
Mittag T, Parker R. Mittag T, et al. J Mol Biol. 2018 Nov 2;430(23):4636-4649. doi: 10.1016/j.jmb.2018.08.005. Epub 2018 Aug 9. J Mol Biol. 2018. PMID: 30099026 Free PMC article. Review. - Multicolour single-molecule tracking of mRNA interactions with RNP granules.
Moon SL, Morisaki T, Khong A, Lyon K, Parker R, Stasevich TJ. Moon SL, et al. Nat Cell Biol. 2019 Feb;21(2):162-168. doi: 10.1038/s41556-018-0263-4. Epub 2019 Jan 21. Nat Cell Biol. 2019. PMID: 30664789 Free PMC article. - Eukaryotic stress granules: the ins and outs of translation.
Buchan JR, Parker R. Buchan JR, et al. Mol Cell. 2009 Dec 25;36(6):932-41. doi: 10.1016/j.molcel.2009.11.020. Mol Cell. 2009. PMID: 20064460 Free PMC article. Review. - Mechanistic insights into mammalian stress granule dynamics.
Panas MD, Ivanov P, Anderson P. Panas MD, et al. J Cell Biol. 2016 Nov 7;215(3):313-323. doi: 10.1083/jcb.201609081. J Cell Biol. 2016. PMID: 27821493 Free PMC article. Review.
Cited by
- Emerging roles of liquid-liquid phase separation in liver innate immunity.
Zhang X, Yang Z, Fu C, Yao R, Li H, Peng F, Li N. Zhang X, et al. Cell Commun Signal. 2024 Sep 3;22(1):430. doi: 10.1186/s12964-024-01787-4. Cell Commun Signal. 2024. PMID: 39227829 Free PMC article. Review. - A Comprehensive Analysis of the Role of hnRNP A1 Function and Dysfunction in the Pathogenesis of Neurodegenerative Disease.
Clarke JP, Thibault PA, Salapa HE, Levin MC. Clarke JP, et al. Front Mol Biosci. 2021 Apr 12;8:659610. doi: 10.3389/fmolb.2021.659610. eCollection 2021. Front Mol Biosci. 2021. PMID: 33912591 Free PMC article. Review. - Identification of a non-canonical G3BP-binding sequence in a Mayaro virus nsP3 hypervariable domain.
Neyret A, Bernard E, Aïqui-Reboul-Paviet O, Bakhache W, Eldin P, Chaloin L, Briant L. Neyret A, et al. Front Cell Infect Microbiol. 2022 Aug 11;12:958176. doi: 10.3389/fcimb.2022.958176. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36034716 Free PMC article. - Pharmacological inhibition of DEAD-Box RNA Helicase 3 attenuates stress granule assembly.
Cui BC, Sikirzhytski V, Aksenova M, Lucius MD, Levon GH, Mack ZT, Pollack C, Odhiambo D, Broude E, Lizarraga SB, Wyatt MD, Shtutman M. Cui BC, et al. Biochem Pharmacol. 2020 Dec;182:114280. doi: 10.1016/j.bcp.2020.114280. Epub 2020 Oct 10. Biochem Pharmacol. 2020. PMID: 33049245 Free PMC article. - eIF4G1 N-terminal intrinsically disordered domain is a multi-docking station for RNA, Pab1, Pub1, and self-assembly.
Chaves-Arquero B, Martínez-Lumbreras S, Sibille N, Camero S, Bernadó P, Jiménez MÁ, Zorrilla S, Pérez-Cañadillas JM. Chaves-Arquero B, et al. Front Mol Biosci. 2022 Sep 23;9:986121. doi: 10.3389/fmolb.2022.986121. eCollection 2022. Front Mol Biosci. 2022. PMID: 36213119 Free PMC article.
References
- Spector DL. SnapShot: Cellular Bodies. Cell. 2006 Dec;127(5):1071.e1–1071.e2. - PubMed
- Barbarese E, Ifrim MF, Hsieh L, Guo C, Tatavarty V, Maggipinto MJ, et al. Conditional Knockout of Tumor Overexpressed Gene in Mouse Neurons Affects RNA Granule Assembly, Granule Translation, LTP and Short Term Habituation. In: Baudry M, editor. PLoS One. 8. Vol. 8. 2013. Aug 6, p. e69989. - PMC - PubMed
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009 Jun 26;324(5935):1729–32. - PubMed
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10(6):430–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources