Epidemiology of a Novel Recombinant Middle East Respiratory Syndrome Coronavirus in Humans in Saudi Arabia - PubMed (original) (raw)
. 2016 Sep 1;214(5):712-21.
doi: 10.1093/infdis/jiw236. Epub 2016 Jun 14.
Claire M Midgley 2, Glen R Abedi 3, Abdulaziz Bin Saeed 4, Malak M Almasri 1, Xiaoyan Lu 3, Hail M Al-Abdely 1, Osman Abdalla 1, Mutaz Mohammed 1, Homoud S Algarni 1, Raafat F Alhakeem 1, Senthilkumar K Sakthivel 3, Randa Nooh 5, Zainab Alshayab 5, Mohammad Alessa 5, Ganesh Srinivasamoorthy 3, Saeed Yahya AlQahtani 1, Ali Kheyami 1, Waleed Husein HajOmar 1, Talib M Banaser 1, Ahmad Esmaeel 1, Aron J Hall 3, Aaron T Curns 3, Azaibi Tamin 3, Ali Abraheem Alsharef 1, Dean Erdman 3, John T Watson 3, Susan I Gerber 3
Affiliations
- PMID: 27302191
- PMCID: PMC5712457
- DOI: 10.1093/infdis/jiw236
Epidemiology of a Novel Recombinant Middle East Respiratory Syndrome Coronavirus in Humans in Saudi Arabia
Abdullah M Assiri et al. J Infect Dis. 2016.
Erratum in
- Erratum.
[No authors listed] [No authors listed] J Infect Dis. 2019 Aug 30;220(7):1235. doi: 10.1093/infdis/jiz255. J Infect Dis. 2019. PMID: 31505660 Free PMC article. No abstract available.
Abstract
Background: Middle East respiratory syndrome coronavirus (MERS-CoV) causes severe respiratory illness in humans. Fundamental questions about circulating viruses and transmission routes remain.
Methods: We assessed routinely collected epidemiologic data for MERS-CoV cases reported in Saudi Arabia during 1 January-30 June 2015 and conducted a more detailed investigation of cases reported during February 2015. Available respiratory specimens were obtained for sequencing.
Results: During the study period, 216 MERS-CoV cases were reported. Full genome (n = 17) or spike gene sequences (n = 82) were obtained from 99 individuals. Most sequences (72 of 99 [73%]) formed a discrete, novel recombinant subclade (NRC-2015), which was detected in 6 regions and became predominant by June 2015. No clinical differences were noted between clades. Among 87 cases reported during February 2015, 13 had no recognized risks for secondary acquisition; 12 of these 13 also denied camel contact. Most viruses (8 of 9) from these 13 individuals belonged to NRC-2015.
Discussions: Our findings document the spread and eventual predominance of NRC-2015 in humans in Saudi Arabia during the first half of 2015. Our identification of cases without recognized risk factors but with similar virus sequences indicates the need for better understanding of risk factors for MERS-CoV transmission.
Keywords: MERS; MERS epidemiology; MERS phylogeny; MERS transmission; Middle East respiratory syndrome; Saudi Arabia; coronavirus; recombinant.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
Figure 1.
Phylogeny of Middle East respiratory syndrome coronavirus (MERS-CoV) spike sequences. Phylogenetic analysis of the MERS-CoV spike gene coding region (4062 nucleotides) obtained from 99 cases in this study and 251 previously published sequences available in GenBank. Major clades A and B are indicated by vertical bars. Figure 1 continued. The novel subclade (NRC-2015) is repositioned for clarity. Spike gene sequences obtained in this study are marked with solid circles; red circles indicate samples with available genome sequences (Supplementary Figure 1). The tree was constructed by the neighbor-joining method, and bootstrap resampling values (1000 replicates) ≥70% are indicated above the respective nodes. Numbers in brackets following some strain identifiers are the number of identical sequences with the same location and sample collection time. The scale bar shows the genetic distance as nucleotide substitutions per site. To compare persistence and geographic distribution of NRC-2015 with other viruses identified during 2012–2015, we assigned virus sequences to different subclades (depicted in color), based on previously described clades [19, 28–30].
Figure 2.
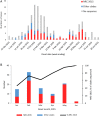
Middle East respiratory syndrome coronavirus (MERS-CoV) cases reported by the Saudi Arabia Ministry of Health (MoH) during 1 January–30 June 2015. A, MERS-CoV cases, by clade and week of illness onset. B, Sequenced viruses, by clade and month of illness onset. The 216 cases reported by the MoH during 1 January–30 June 2015 are included. These graphs do not include the single sequence from the individual with no recognized symptoms who did not meet the case definition. NRC-2015 was defined using the spike gene phylogenies (Figure 1).
Figure 3.
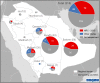
Map of Middle East respiratory syndrome coronavirus (MERS-CoV) cases, by subclade and region of Saudi Arabia. The 216 cases reported by the Saudi Arabia Ministry of Health during 1 January–30 June 2015, are included. This map does not include the single sequence from the individual with no recognized symptoms who did not meet the case definition. NRC-2015 was defined using the spike gene phylogenies (Figure 1).
Figure 4.
Geotemporal distribution of Middle East respiratory syndrome coronavirus (MERS-CoV) clades in Saudi Arabia during 1 January 2013–30 June 2015. For human-derived sequences in each subclade within the broader clade B (as defined using the spike gene phylogenetic analysis in Figure 1), we used GenBank to determine length of persistence, using the earliest and most recent date of detection (A), and we determined geographic distribution, based on city of detection within Saudi Arabia (B). a2015 depicts clades detected through 30 June 2015.
Similar articles
- Genomic Sequencing and Analysis of Eight Camel-Derived Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Isolates in Saudi Arabia.
Al-Shomrani BM, Manee MM, Alharbi SN, Altammami MA, Alshehri MA, Nassar MS, Bakhrebah MA, Al-Fageeh MB. Al-Shomrani BM, et al. Viruses. 2020 Jun 3;12(6):611. doi: 10.3390/v12060611. Viruses. 2020. PMID: 32503352 Free PMC article. - Genetic diversity of MERS-CoV spike protein gene in Saudi Arabia.
Sohrab SS, Azhar EI. Sohrab SS, et al. J Infect Public Health. 2020 May;13(5):709-717. doi: 10.1016/j.jiph.2019.11.007. Epub 2019 Dec 9. J Infect Public Health. 2020. PMID: 31831395 Free PMC article. - Phylogenetic Analysis of MERS-CoV in a Camel Abattoir, Saudi Arabia, 2016-2018.
Hemida MG, Chu DKW, Chor YY, Cheng SMS, Poon LLM, Alnaeem A, Peiris M. Hemida MG, et al. Emerg Infect Dis. 2020 Dec;26(12):3089-3091. doi: 10.3201/eid2612.191094. Emerg Infect Dis. 2020. PMID: 33219804 Free PMC article. - MERS-CoV: epidemiology, molecular dynamics, therapeutics, and future challenges.
Rabaan AA, Al-Ahmed SH, Sah R, Alqumber MA, Haque S, Patel SK, Pathak M, Tiwari R, Yatoo MI, Haq AU, Bilal M, Dhama K, Rodriguez-Morales AJ. Rabaan AA, et al. Ann Clin Microbiol Antimicrob. 2021 Jan 18;20(1):8. doi: 10.1186/s12941-020-00414-7. Ann Clin Microbiol Antimicrob. 2021. PMID: 33461573 Free PMC article. Review. - Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission.
Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Hui DS, et al. Lancet Infect Dis. 2018 Aug;18(8):e217-e227. doi: 10.1016/S1473-3099(18)30127-0. Epub 2018 Apr 18. Lancet Infect Dis. 2018. PMID: 29680581 Free PMC article. Review.
Cited by
- Molecular aspects of MERS-CoV.
Rabaan AA, Bazzi AM, Al-Ahmed SH, Al-Tawfiq JA. Rabaan AA, et al. Front Med. 2017 Sep;11(3):365-377. doi: 10.1007/s11684-017-0521-z. Epub 2017 May 13. Front Med. 2017. PMID: 28500431 Free PMC article. Review. - Genomic Diversity and Recombination Analysis of the Spike Protein Gene from Selected Human Coronaviruses.
Sohrab SS, Alsaqaf F, Hassan AM, Tolah AM, Bajrai LH, Azhar EI. Sohrab SS, et al. Biology (Basel). 2024 Apr 22;13(4):282. doi: 10.3390/biology13040282. Biology (Basel). 2024. PMID: 38666894 Free PMC article. - Evolving sequence mutations in the Middle East Respiratory Syndrome Coronavirus (MERS-CoV).
AlBalwi MA, Khan A, AlDrees M, Gk U, Manie B, Arabi Y, Alabdulkareem I, AlJohani S, Alghoribi M, AlAskar A, AlAjlan A, Hajeer A. AlBalwi MA, et al. J Infect Public Health. 2020 Oct;13(10):1544-1550. doi: 10.1016/j.jiph.2020.06.030. Epub 2020 Jul 1. J Infect Public Health. 2020. PMID: 32654959 Free PMC article. - Infectious MERS-CoV Isolated From a Mildly Ill Patient, Saudi Arabia.
Al-Abdely HM, Midgley CM, Alkhamis AM, Abedi GR, Tamin A, Binder AM, Alanazi K, Lu X, Abdalla O, Sakthivel SK, Mohammed M, Queen K, Algarni HS, Li Y, Trivedi S, Algwizani A, Alhakeem RF, Thornburg NJ, Tong S, Ghazal SS, Erdman DD, Assiri AM, Gerber SI, Watson JT. Al-Abdely HM, et al. Open Forum Infect Dis. 2018 May 15;5(6):ofy111. doi: 10.1093/ofid/ofy111. eCollection 2018 Jun. Open Forum Infect Dis. 2018. PMID: 30294617 Free PMC article. - Middle East Respiratory Syndrome Coronavirus Infection Dynamics and Antibody Responses among Clinically Diverse Patients, Saudi Arabia.
Al-Abdely HM, Midgley CM, Alkhamis AM, Abedi GR, Lu X, Binder AM, Alanazi KH, Tamin A, Banjar WM, Lester S, Abdalla O, Dahl RM, Mohammed M, Trivedi S, Algarni HS, Sakthivel SK, Algwizani A, Bafaqeeh F, Alzahrani A, Alsharef AA, Alhakeem RF, Jokhdar HAA, Ghazal SS, Thornburg NJ, Erdman DD, Assiri AM, Watson JT, Gerber SI. Al-Abdely HM, et al. Emerg Infect Dis. 2019 Apr;25(4):753-766. doi: 10.3201/eid2504.181595. Emerg Infect Dis. 2019. PMID: 30882305 Free PMC article.
References
- World Health Organization. Summary of current situation, literature update and risk assessment—7 July 2015. http://apps.who.int/iris/bitstream/10665/179184/2/WHO_MERS_RA_15.1_eng.pdf Accessed 4 October 2015.
- Azhar EI, El-Kafrawy SA, Farraj SA et al. . Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–505. - PubMed
- Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill 2014; 19:20781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources