Adolescent Alcohol Exposure: Burden of Epigenetic Reprogramming, Synaptic Remodeling, and Adult Psychopathology - PubMed (original) (raw)
Review
Adolescent Alcohol Exposure: Burden of Epigenetic Reprogramming, Synaptic Remodeling, and Adult Psychopathology
Evan J Kyzar et al. Front Neurosci. 2016.
Abstract
Adolescence represents a crucial phase of synaptic maturation characterized by molecular changes in the developing brain that shape normal behavioral patterns. Epigenetic mechanisms play an important role in these neuromaturation processes. Perturbations of normal epigenetic programming during adolescence by ethanol can disrupt these molecular events, leading to synaptic remodeling and abnormal adult behaviors. Repeated exposure to binge levels of alcohol increases the risk for alcohol use disorder (AUD) and comorbid psychopathology including anxiety in adulthood. Recent studies in the field clearly suggest that adolescent alcohol exposure causes widespread and persistent changes in epigenetic, neurotrophic, and neuroimmune pathways in the brain. These changes are manifested by altered synaptic remodeling and neurogenesis in key brain regions leading to adult psychopathology such as anxiety and alcoholism. This review details the molecular mechanisms underlying adolescent alcohol exposure-induced changes in synaptic plasticity and the development of alcohol addiction-related phenotypes in adulthood.
Keywords: adolescence; anxiety; binge drinking; dendritic spines; epigenetics; neurogenesis; neuroinflammation.
Figures
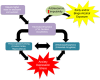
Figure 1
Alcohol use disorder is characterized by the classical pattern of addiction, namely a cycle from a state of euphoria during alcohol intoxication to that of dysphoria during alcohol withdrawal to one of craving in the absence of acute intoxication. Craving, with or without withdrawal symptoms, is characterized by preoccupation with obtaining alcohol and anticipation of alcohol use, leading to relapse and a return to the intoxicated state. Certain individual clinical characteristics or psychosocial factors can exacerbate this cycle, driving alcohol use and addiction. For example, impulsivity renders an individual more sensitive to the immediate, rewarding effects of alcohol intake, with minimization of any longer term negative consequences, driving alcohol intake. Adolescence alone is characterized by an increased sensitivity to the rewarding effects of alcohol with a protection from the negative effects relative to aged counterparts, driving alcohol intake for this group. Adolescence is often characterized by impulsivity and together these characteristics facilitate alcohol intake. Also they require higher doses of ethanol to produce anxiolysis and sedation. Withdrawal from alcohol can both induce anxiety or depression symptoms and be worsened by comorbid mood disorders. Anxiety or depression can separately be exacerbated by other stressors, acute or chronic, and has been shown to be increased over the long term by environmental insults during development (early adversity). Stress and/or mood decompensation can also, in the absence of withdrawal dysphoria, directly stimulate craving and relapse, driving the cycle of addiction at either stage.
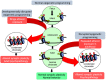
Figure 2
Hypothetical model of epigenetic reprogramming by alcohol during adolescence. Adolescence is characterized by widespread changes in the developing brain. These changes are underpinned by molecular processes such as epigenetic programming, which turn specific genetic programs on to mediate cell fate determination, axonal outgrowth, dendrite formation and neuronal maturation. These processes, in turn, affect synaptic plasticity and neurocircuit function to shape normal behaviors. However, developmental perturbation by early life and/or binge alcohol exposure during this critical period can produce persistent effects on chromatin remodeling, synaptic plasticity, and brain function in adulthood, leading to alcohol use disorders (AUDs) and comorbid psychopathology including anxiety and depression. Adult exposure of alcohol can also disturb chromatin remodeling and plasticity, but some of these effects may not be long-lasting.
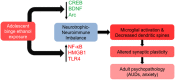
Figure 3
Molecular and cellular signaling mechanisms in the brain leading to altered synaptic plasticity in adulthood after adolescent alcohol exposure. Adolescent binge alcohol exposure exerts effects in the brain long after alcohol has left the system. For example, adolescent alcohol causes a long-lasting decrease in key synaptic plasticity-related genes including cyclic AMP response element binding protein (CREB) pathway (CREB binding protein), brain-derived neurotrophic factor (BDNF), and activity-regulated cytoskeleton-associated protein (Arc). At the same time, adolescent binge alcohol exposure increases neuroimmune mediators including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), high mobility group box 1 (HMGB1) and toll-like receptor 4 (TLR4). The increase in neuroimmune factors and decrease in neurotrophic factors may be responsible for molecular imbalance in the adult brain that is mediated by increased microglial activation and decreased synaptic plasticity, leading to an increased risk for alcohol use disorders (AUDs) and related psychopathology such as anxiety.
Similar articles
- MicroRNA-137 Drives Epigenetic Reprogramming in the Adult Amygdala and Behavioral Changes after Adolescent Alcohol Exposure.
Kyzar EJ, Bohnsack JP, Zhang H, Pandey SC. Kyzar EJ, et al. eNeuro. 2019 Dec 3;6(6):ENEURO.0401-19.2019. doi: 10.1523/ENEURO.0401-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31740576 Free PMC article. - Targeting Persistent Changes in Neuroimmune and Epigenetic Signaling in Adolescent Drinking to Treat Alcohol Use Disorder in Adulthood.
Crews FT, Coleman LG Jr, Macht VA, Vetreno RP. Crews FT, et al. Pharmacol Rev. 2023 Mar;75(2):380-396. doi: 10.1124/pharmrev.122.000710. Epub 2022 Dec 12. Pharmacol Rev. 2023. PMID: 36781218 Free PMC article. Review. - Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings.
Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, Vetreno RP. Crews FT, et al. Alcohol Clin Exp Res. 2019 Sep;43(9):1806-1822. doi: 10.1111/acer.14154. Epub 2019 Aug 14. Alcohol Clin Exp Res. 2019. PMID: 31335972 Free PMC article. Review. - Epigenetic regulation of microglia and neurons by proinflammatory signaling following adolescent intermittent ethanol (AIE) exposure and in human AUD.
Crews FT, Macht V, Vetreno RP. Crews FT, et al. Adv Drug Alcohol Res. 2024 Mar 8;4:12094. doi: 10.3389/adar.2024.12094. eCollection 2024. Adv Drug Alcohol Res. 2024. PMID: 38524847 Free PMC article. Review. - Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article.
Cited by
- Behavioral and Amygdala Biochemical Damage Induced by Alternating Mild Stress and Ethanol Intoxication in Adolescent Rats: Reversal by Argan Oil Treatment?
El Mostafi H, Elhessni A, Doumar H, Touil T, Mesfioui A. El Mostafi H, et al. Int J Mol Sci. 2024 Sep 30;25(19):10529. doi: 10.3390/ijms251910529. Int J Mol Sci. 2024. PMID: 39408860 Free PMC article. - Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal.
George O, Hope BT. George O, et al. Neuropharmacology. 2017 Aug 1;122:107-114. doi: 10.1016/j.neuropharm.2017.04.031. Epub 2017 Apr 20. Neuropharmacology. 2017. PMID: 28435008 Free PMC article. Review. - Alcohol and lactation: Developmental deficits in a mouse model.
Perez RF Jr, Conner KE, Erickson MA, Nabatanzi M, Huffman KJ. Perez RF Jr, et al. Front Neurosci. 2023 Mar 13;17:1147274. doi: 10.3389/fnins.2023.1147274. eCollection 2023. Front Neurosci. 2023. PMID: 36992847 Free PMC article. - Current and Future Perspectives of Noncoding RNAs in Brain Function and Neuropsychiatric Disease.
Kyzar EJ, Bohnsack JP, Pandey SC. Kyzar EJ, et al. Biol Psychiatry. 2022 Jan 15;91(2):183-193. doi: 10.1016/j.biopsych.2021.08.013. Epub 2021 Aug 24. Biol Psychiatry. 2022. PMID: 34742545 Free PMC article. Review. - Effects of adolescent alcohol consumption on the brain and behaviour.
Spear LP. Spear LP. Nat Rev Neurosci. 2018 Apr;19(4):197-214. doi: 10.1038/nrn.2018.10. Epub 2018 Feb 15. Nat Rev Neurosci. 2018. PMID: 29467469 Review.
References
- Alaux-Cantin S., Warnault V., Legastelois R., Botia B., Pierrefiche O., Vilpoux C., et al. . (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67, 521–531. 10.1016/j.neuropharm.2012.12.007 - DOI - PubMed
- Balosso S., Liu J., Bianchi M. E., Vezzani A. (2014). Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid. Redox Signal. 21, 1726–1740. 10.1089/ars.2013.5349 - DOI - PubMed
Publication types
Grants and funding
- I01 BX000143/BX/BLRD VA/United States
- U01 AA019971/AA/NIAAA NIH HHS/United States
- R01 AA010005/AA/NIAAA NIH HHS/United States
- U24 AA024605/AA/NIAAA NIH HHS/United States
- P50 AA022538/AA/NIAAA NIH HHS/United States
- R01 AA013341/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources