Vitamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic α-Tocopherol Transfer Protein - PubMed (original) (raw)
Vitamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic α-Tocopherol Transfer Protein
Stacey Chung et al. J Biol Chem. 2016.
Abstract
α-Tocopherol (vitamin E) is an essential nutrient for all vertebrates. From the eight naturally occurring members of the vitamin E family, α-tocopherol is the most biologically active species and is selectively retained in tissues. The hepatic α-tocopherol transfer protein (TTP) preferentially selects dietary α-tocopherol and facilitates its transport through the hepatocyte and its secretion to the circulation. In doing so, TTP regulates body-wide levels of α-tocopherol. The mechanisms by which TTP facilitates α-tocopherol trafficking in hepatocytes are poorly understood. We found that the intracellular localization of TTP in hepatocytes is dynamic and responds to the presence of α-tocopherol. In the absence of the vitamin, TTP is localized to perinuclear vesicles that harbor CD71, transferrin, and Rab8, markers of the recycling endosomes. Upon treatment with α-tocopherol, TTP- and α-tocopherol-containing vesicles translocate to the plasma membrane, prior to secretion of the vitamin to the exterior of the cells. The change in TTP localization is specific to α-tocopherol and is time- and dose-dependent. The aberrant intracellular localization patterns of lipid binding-defective TTP mutants highlight the importance of protein-lipid interaction in the transport of α-tocopherol. These findings provide the basis for a proposed mechanistic model that describes TTP-facilitated trafficking of α-tocopherol through hepatocytes.
Keywords: lipid trafficking; lipid transport; phosphatidylinositol signaling; phosphoinositide; tocopherol; tocopherol transfer protein; vitamin E.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
Localization of NBD-α-tocopherol during uptake into hepatocytes. McARH7777 cells were incubated with serum-complexed NBD-α-tocopherol at 4 °C for 1 h prior to washing and incubation at 37 °C for the indicated times. Live cells were co-stained with 100 n
m
LysoTracker Red (A) or 100 n
m
Nile Red (B) and visualized by confocal fluorescence microscopy. NBD-α-tocopherol is shown in green, and LysoTracker and Nile red are shown in red. Arrows indicate sites of co-localization. Scale bar, 10 μm.
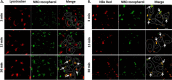
FIGURE 2.
Intracellular localization of TTP is regulated by vitamin E. Because established hepatocyte cell lines do not endogenously express TTP, we utilized transient transfection to express the human TTPA gene in McARH7777 cells. A–C, indicated TTP variant was transiently transfected (in the pCDNA3.1 vector) into vitamin E-depleted McARH7777 cells. Where indicated, serum-complexed α-tocopherol (35 μ
m
) was added to the cultures for 24 h prior to fixing, immunostaining, and imaging by confocal fluorescence microscopy. D, RFP-tagged TTP-expressing (in the pTagRFP plasmid; left) or RFP-expressing (right) plasmids were transiently transfected and treated as in A, and protein localization was determined by live-cell fluorescence microscopy. E, kinetics of α-tocopherol-induced shift in TTP's localization. Localization of TTP was determined at different times after addition of 35 μ
m
serum-complexed α-tocopherol as in D, and fraction of the cells in which the protein displayed a punctate pattern was quantified by visual inspection of fluorescence micrographs (total of 300–400 cells in each of four images per condition). Shown are averages and standard deviations in one representative of three independent experiments. F, dose-response of α-tocopherol-induced shift in TTP's localization. Fraction of cells in which the protein displayed a punctate pattern was quantified 24 h after treatment with the indicated concentration of serum-complexed α-tocopherol (or cholesterol) as in E. Asterisks indicate statistically significant difference from control (p < 0.05), determined by Student's t test.
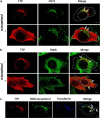
FIGURE 3.
TTP localizes to the recycling endosomes. McARH7777 cells stably expressing pTRE2-TTP and induced with doxycycline for 48 h were fixed and immunostained with antibodies directed against TTP (red), the transferrin receptor (A; green), or Rab8 (B; green). Where indicated, serum-complexed α-tocopherol was added to 35 μ
m
for 48 h prior to fixing, immunostaining, and visualization under the confocal fluorescence microscope. C, McARH7777 cells were incubated with Alexa Fluor 633-labeled transferrin (blue) and NBD-tocopherol (green) at 4 °C for 1 h prior to incubation at 37 °C for 30 min, fixing and visualization with confocal fluorescence microscopy. White arrows indicate sites of co-localization. Data are representative of three independent experiments. Scale bars, 5 μm.
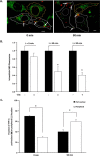
FIGURE 4.
Time-dependent changes in the intracellular localization of NBD-α-tocopherol and TTP. McARH7777 cells were transiently transfected with pTagRFP-TTP. Forty eight hours after transfection, cells were pulse-loaded with 10 μ
m
serum-complexed NBD-α-tocopherol, and live confocal images of NBD-α-tocopherol (green) or TTP (red) were taken at the indicated times. A, representative images showing cells that do (white outline) or do not (yellow outline) express TTP. Scale bars, 5 μm. B, quantification of intracellular NBD-α-tocopherol fluorescence over time in cells that do (white bars) or do not (black bars) express TTP. Shown are averages and standard errors of NBD-α-tocopherol fluorescence quantified in confocal images of ∼50 cells of each group from three independent experiments. Asterisks denote statistical significance with p < 0.01, calculated by Student's t test. Fluorescence intensities were normalized to the values of non-TTP-expressing cells. C, time-dependent changes in the intracellular localization of TTP. Localization of TTP in perinuclear (black bars) or peripheral (white bars) cellular locations during secretion of NBD-α-tocopherol was manually determined in three images (20–50 cells) of each condition. Asterisks denote statistically significant differences determined by Student's t test (p < 0.01). Data are representative of three independent experiments.
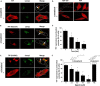
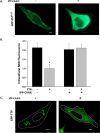
FIGURE 5.
Depletion of plasma membrane PI(4,5)P2 inhibits TTP function. McARH7777 cells stably expressing pTagRFP-TTP were grown without vitamin E as in Fig. 2. Where indicated, cells were also transiently transfected with membrane-targeted inositol 5′-phosphatase domain of synaptojanin 1 encoded in the pcDNA3-HA vector (IPP-C_AAX_ (55–58)). Cells were then pulse-loaded with 10 μ
m
serum-complexed NBD-α-tocopherol as described under “Experimental Procedures” and imaged by fluorescence microscopy. A, depletion of plasma membrane PI(4,5)P2 by IPP-C_AAX_. Cells were co-transfected with a construct encoding pEGFP-tagged PH domain of PLCδ. Left panel, localization of the PH domain is restricted to the cells' plasma membrane (79). Right panel, upon expression IPP-C_AAX_, the PH domain is no longer at the plasma membrane, indicating depletion of membrane PI(4,5)P2 (55–58). Scale bar, 5 μm. B, TTP-mediated secretion of α-tocopherol is dependent upon plasma membrane PI(4,5)P2. Secretion of NBD-α-tocopherol in TTP-expressing cells was quantified as in Fig. 4. Where indicated, cells were also transfected with pCDNA3-encoded IPP-C_AAX. Asterisk_ denotes statistical significance with p < 0.01, calculated by Student's t test. C, depletion of plasma membrane PI(4,5)P2 causes mislocalization of TTP. McARH7777 cells were transiently co-transfected with plasmids encoding GFP-tagged TTP and IPP-C_AAX_ (or empty vector) prior to live cell confocal fluorescence imaging as in Fig. 2_D. Scale bar_, 5 μm. Figure is representative of three independent experiments.
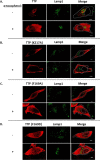
FIGURE 6.
Roles of protein-lipid interactions on the intracellular localization of TTP: substitutions of Phe169 and Lys217 affect TTP localization. McARH7777 cells were transiently transfected with the indicated variant of TTP and treated with serum-complexed 35 μ
m
α-tocopherol as in Fig. 2. Cells were fixed and immunostained using anti-TTP (red) and anti-Lamp1 (green) antibodies, prior to visualization with confocal fluorescence microscopy. Scale bar, 5 μm.
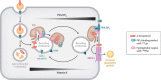
FIGURE 7.
Proposed model of TTP-facilitated vitamin E trafficking in hepatocytes. TTP initially associates with endocytic vesicles through the hydrophobic surface that contains Phe169. After uptake through receptor-mediated endocytosis, recycling endosomes that contain the transferrin receptor, Rab8, TTP and vitamin E then travel to the plasma membrane, where the vesicles “dock” at PI(4,5)P2-rich domains. Binding of PI(4,5)P2 to TTP's positively charged “patch” that includes Lys217 causes a conformational change in TTP, leading to release of the protein-bound vitamin E to the plasma membrane. Subsequently, TTP cycles back to vitamin E-containing organelles to repeat this cycle of regulated vitamin E secretion. Directionality of the transport process is provided by the opposite concentration gradients of PI(4,5)P2 (high at the plasma membrane) and α-tocopherol (high at the endocytic compartment). After release to the plasma membrane, vitamin E exits through an ABC-type transporter and associates with lipoprotein particles that deliver it to extra-hepatic tissues. Adapted from Ref. with the author's permission.
Similar articles
- pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes.
Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. Horiguchi M, et al. Genes Cells. 2003 Oct;8(10):789-800. doi: 10.1046/j.1365-2443.2003.00676.x. Genes Cells. 2003. PMID: 14531858 - Hepatic α-tocopherol transfer protein: ligand-induced protection from proteasomal degradation.
Thakur V, Morley S, Manor D. Thakur V, et al. Biochemistry. 2010 Nov 2;49(43):9339-44. doi: 10.1021/bi100960b. Biochemistry. 2010. PMID: 20828164 Free PMC article. - Biochemical consequences of heritable mutations in the alpha-tocopherol transfer protein.
Qian J, Atkinson J, Manor D. Qian J, et al. Biochemistry. 2006 Jul 11;45(27):8236-42. doi: 10.1021/bi060522c. Biochemistry. 2006. PMID: 16819822 - α-Tocopherol transfer protein (α-TTP).
Arai H, Kono N. Arai H, et al. Free Radic Biol Med. 2021 Nov 20;176:162-175. doi: 10.1016/j.freeradbiomed.2021.09.021. Epub 2021 Sep 24. Free Radic Biol Med. 2021. PMID: 34563650 Review. - Vitamin E trafficking.
Traber MG, Burton GW, Hamilton RL. Traber MG, et al. Ann N Y Acad Sci. 2004 Dec;1031:1-12. doi: 10.1196/annals.1331.001. Ann N Y Acad Sci. 2004. PMID: 15753129 Review.
Cited by
- Vitamin E Supplementation and Mitochondria in Experimental and Functional Hyperthyroidism: A Mini-Review.
Napolitano G, Fasciolo G, Di Meo S, Venditti P. Napolitano G, et al. Nutrients. 2019 Dec 1;11(12):2900. doi: 10.3390/nu11122900. Nutrients. 2019. PMID: 31805673 Free PMC article. Review. - Steatitis in Cold-Stunned Kemp's Ridley Sea Turtles (Lepidochelys kempii).
Turner RC, Innis CJ, Stacy BA, Hernandez JA, Hill RC, Scott KC, Frasca S Jr, Garner MM, Burns RE, Arendt MD, Brisson J, Norton TM, Williams SR, Kennedy A, Alexander AB, Stacy NI. Turner RC, et al. Animals (Basel). 2021 Mar 21;11(3):898. doi: 10.3390/ani11030898. Animals (Basel). 2021. PMID: 33801097 Free PMC article. - RedEfish: Generation of the Polycistronic mScarlet: GSG-T2A: Ttpa Zebrafish Line.
Head B, La Du J, Barton C, Zhang J, Wong C, Ho E, Tanguay RL, Traber MG. Head B, et al. Antioxidants (Basel). 2021 Jun 16;10(6):965. doi: 10.3390/antiox10060965. Antioxidants (Basel). 2021. PMID: 34208660 Free PMC article. - Self-assembled α-Tocopherol Transfer Protein Nanoparticles Promote Vitamin E Delivery Across an Endothelial Barrier.
Aeschimann W, Staats S, Kammer S, Olieric N, Jeckelmann JM, Fotiadis D, Netscher T, Rimbach G, Cascella M, Stocker A. Aeschimann W, et al. Sci Rep. 2017 Jul 10;7(1):4970. doi: 10.1038/s41598-017-05148-9. Sci Rep. 2017. PMID: 28694484 Free PMC article. - Vitamin E sequestration by liver fat in humans.
Violet PC, Ebenuwa IC, Wang Y, Niyyati M, Padayatty SJ, Head B, Wilkins K, Chung S, Thakur V, Ulatowski L, Atkinson J, Ghelfi M, Smith S, Tu H, Bobe G, Liu CY, Herion DW, Shamburek RD, Manor D, Traber MG, Levine M. Violet PC, et al. JCI Insight. 2020 Jan 16;5(1):e133309. doi: 10.1172/jci.insight.133309. JCI Insight. 2020. PMID: 31821172 Free PMC article. Clinical Trial.
References
- Evans H. M., and Bishop K. S. (1922) On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56, 650–651 - PubMed
- Ulatowski L. M., and Manor D. (2015) Vitamin E and neurodegeneration. Neurobiol. Dis. 84, 78–83 - PubMed
- DellaPenna D., and Pogson B. J. (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 57, 711–738 - PubMed
- Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., and Inoue K. (1997) Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 409, 105–108 - PubMed
- Leth T., and Sondergaard H. (1977) Biological activity of vitamin E compounds and natural materials by the resorption-gestation test, and chemical determination of the vitamin E activity in foods and feeds. J. Nutr. 107, 2236–2243 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials