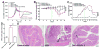Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer - PubMed (original) (raw)
Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer
Mingzhen Zhang et al. Biomaterials. 2016 Sep.
Abstract
There is a clinical need for new, more effective treatments for chronic and debilitating inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis. In this study, we characterized a specific population of nanoparticles derived from edible ginger (GDNPs 2) and demonstrated their efficient colon targeting following oral administration. GDNPs 2 had an average size of ∼230 nm and exhibited a negative zeta potential. These nanoparticles contained high levels of lipids, a few proteins, ∼125 microRNAs (miRNAs), and large amounts of ginger bioactive constituents (6-gingerol and 6-shogaol). We also demonstrated that GDNPs 2 were mainly taken up by intestinal epithelial cells (IECs) and macrophages, and were nontoxic. Using different mouse colitis models, we showed that GDNPs 2 reduced acute colitis, enhanced intestinal repair, and prevented chronic colitis and colitis-associated cancer (CAC). 2D-DIGE/MS analyses further identified molecular target candidates of GDNPs 2 involved in these mouse models. Oral administration of GDNPs 2 increased the survival and proliferation of IECs and reduced the pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), and increased the anti-inflammatory cytokines (IL-10 and IL-22) in colitis models, suggesting that GDNPs 2 has the potential to attenuate damaging factors while promoting the healing effect. In conclusion, GDNPs 2, nanoparticles derived from edible ginger, represent a novel, natural delivery mechanism for improving IBD prevention and treatment with an added benefit of overcoming limitations such as potential toxicity and limited production scale that are common with synthetic nanoparticles.
Keywords: Colitis-associated cancer; Edible ginger derived nanoparticles; Inflammatory bowel disease; Natural drug delivery system; Therapy.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Characterization of GDNPs. (A) Three bands formed after sucrose gradient ultracentrifugation. Band 1 from the 8/30% interface (GDNPs 1) was visualized by TEM (B) and AFM (C). Band 2 from the 30/45% interface (GDNPs 2) was visualized by TEM (D) and AFM (E).
Fig. 2
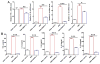
Evaluation of the contents of 6-gingerol and 6-shogaol in ginger-derived nanoparticles (GDNPs) using HPLC/MS. Quantification of 6-gingerol and 6-shogaol in ginger derived nanopaticles, GDNPs 1 and GDNPs 2. The presence of GDNPs 1 and GDNPs 2 were confirmed by using standards and quantified using calibration curve for each individual component. (n=3).
Fig. 3
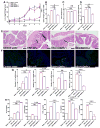
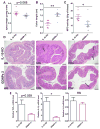
The effect of orally administered GDNPs 2 on the susceptibility of mice to DSS-induced colitis. (A) Lcn-2 level. (B) Spleen/body weight. (C) Colon length. (D) Quantification of colonic MPO activity in the distal colon. (E) Representative H&E-stained colons. Inflammatory cells in the lamina propria are indicated by arrowheads. (F) Immunofluorescence staining for E-cadherin in representative inflamed areas of the colon. (G) Colonic levels of cytokine mRNAs were quantified by real-time RT-PCR and normalized with respect to the mRNA level of the ribosomal protein, 36B4. (H) Protein levels of colon-secreted cytokines were quantified by ELISA. For all panels: *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant; scale bar = 100 μm; n=5.
Fig. 4
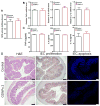
Assess the biocompatibility of GDNPs in vitro. (A) MTT cell proliferation assay was used to assess the potential toxicity of GDNPs 2 in colon-26 and RAW 264.7 macrophage-like cell lines. (B) Barrier function assay was used to determine the influence of GDNPs 2 to the barrier function on caco2-BBE monolayer. (C) At the end of barrier function assay, PBS treated cells were stained with phalloidin-TRITC. Scale Bar=20 μm. (D) At the end of barrier function assay, GDNPs 2 (100 μg/mL) treated cells were stained with phalloidin-TRITC. Scale Bar=20 μm. (E) Cytotoxicity effect of GDNPs 2 on colon-26 cells and RAW 264.7 mouse macrophages after 24 h incubation were measured by FACS. Colon-26 and RAW 264.7 cells were incubated with indicated concentrations of GDNPs 2 for 24 h and then stained with Annexin-V/PI to detect the cell death. Lower left, viable cells (Annexin-V−/PI−); lower right, early apoptotic cells (Annexin-V+/PI−); upper left, necrotic cells (Annexin-V−/PI+); upper right, late apoptotic cells (Annexin-V+/PI+). (n=3).
Fig. 5
Oral administration of GDNPs does not induce side effects at the local or systemic level. Mice (N=5) were oral administrated with GDNPs 2 of 0.3 mg/day for 7 days. (A) Colonic myeloperoxidase (MPO) activity. (B) Quantify proinflammatory cytokines (TNF-α, IL-6 and IL-1β) at mRNA level. (C) Quantify proinflammatory cytokines (TNF-α, IL-6 and IL-1β) at protein level. (D) H&E stain, IEC proliferation and IEC apoptosis examination in colonic tissues. Scale Bar=100 μm. (n=5).
Fig. 6
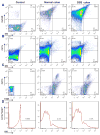
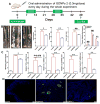
Quantification of uptake efficiency of GDNPs 2 by epithelial cells and macrophages in vivo using flow cytometry. (A) Colonic epithelial cells (EpCAM+), isolated and gated based on EpCAM. (B) Dendritic cells (CD11c+), gated based on CD11c. (C) Macrophages (CD11b+F4/80+), gated based on CD11b and F4/80. (D) DiO-positive cells among Cd11b+F4/80+ macrophages. For control, epithelial cells (EpCAM+), dendritic cells (CD11c+) and macrophages (CD11b+F4/80+) cells were isolated from normal mice without GDNPs 2 oral administration using the same method. (n=3).
Fig. 7
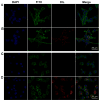
The uptake of GDNPs 2 by epithelial-like (colon-26) and macrophage-like (macrophage 264.7) cells in vitro. (A) Colon-26 cells labeled with DAPI (blue channel) and phalloidin-FITC (green channel). (B) Colon-26 cells incubated with DiL-GDNPs 2 and then labeled with DAPI and phalloidin-FITC. (C) Macrophage cells labeled with DAPI and phalloidin-FITC. (D) Macrophage cells incubated with DiL-GDNPs 2 and then labeled with DAPI and phalloidin-FITC.
Fig. 8
Effect of GDNPs 2 in in vitro and in vivo wound-healing models. (A) GDNPs 2 accelerate healing in wounded intestinal epithelial monolayers using ECIS technology. (B) Body weight changes. (C) Lcn-2 changes. (D) MPO activity changes. (E) H&E-staining. Inflammatory cells in the lamina propria are indicated by arrowheads. Scale bar = 100 μm. (n=5).
Fig. 9
Measurement of mRNA and protein levels of various cytokines in the DSS-induced mouse model of wound healing. (A) Cytokine mRNAs were quantified by real-time PCR. (B) Cytokines proteins were quantified by ELISA kits. *p < 0.05, **p < 0.01, ***p < 0.001. (n=5).
Fig. 10
Effect of GDNPs 2 on chronic colitis in IL10−/− mice. (A) Spleen/body weight. (B) Colon length. (C) MPO activity. (D) Representative H&E-stained colon sections. Inflammatory cells in the lamina propria are indicated by arrowheads. (E) Pro-inflammatory cytokines mRNAs were quantified by real-time RT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. Scale bar = 100 μm. (n=7)
Fig. 11
Effect of GDNPs 2 on colitis-associated cancer (CAC). (A) Protocol for CAC induction. Mice were administered GDNPs 2 (0.3 mg/dose) daily in treatment group. (B) Colon tumor/mouse, tumor load, tumor size and tumor distribution were obtained at the end of the CAC protocol. (C) MPO activities mRNA levels of cytokines and cyclin D1 were quantified. (D) Apoptosis of cells was quantified by TUNEL assay (FITC, green color) and nuclei were stained with DAPI (blue). *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. Scale bar = 100 μm. (n=5).
Similar articles
- Oral delivery of IL-22 mRNA-loaded lipid nanoparticles targeting the injured intestinal mucosa: A novel therapeutic solution to treat ulcerative colitis.
Sung J, Alghoul Z, Long D, Yang C, Merlin D. Sung J, et al. Biomaterials. 2022 Sep;288:121707. doi: 10.1016/j.biomaterials.2022.121707. Epub 2022 Aug 3. Biomaterials. 2022. PMID: 35953326 - Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis.
Zhang M, Xu C, Liu D, Han MK, Wang L, Merlin D. Zhang M, et al. J Crohns Colitis. 2018 Jan 24;12(2):217-229. doi: 10.1093/ecco-jcc/jjx115. J Crohns Colitis. 2018. PMID: 28961808 Free PMC article. - Isolation, Purification, and Characterization of Ginger-derived Nanoparticles (GDNPs) from Ginger, Rhizome of Zingiber officinale.
Sung J, Yang C, Viennois E, Zhang M, Merlin D. Sung J, et al. Bio Protoc. 2019 Oct 5;9(19):e3390. doi: 10.21769/BioProtoc.3390. Bio Protoc. 2019. PMID: 31737748 Free PMC article. - Gingerol and Its Role in Chronic Diseases.
Mohd Yusof YA. Mohd Yusof YA. Adv Exp Med Biol. 2016;929:177-207. doi: 10.1007/978-3-319-41342-6_8. Adv Exp Med Biol. 2016. PMID: 27771925 Review. - Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review.
de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata AMOF, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, Melo-Cavalcante AAC. de Lima RMT, et al. Phytother Res. 2018 Oct;32(10):1885-1907. doi: 10.1002/ptr.6134. Epub 2018 Jul 16. Phytother Res. 2018. PMID: 30009484 Review.
Cited by
- Extracellular Vesicles: Novel Potential Therapeutic Agents in Inflammatory Bowel Diseases.
Mignini I, Piccirilli G, Termite F, Paratore M, Esposto G, Laterza L, Scaldaferri F, Ainora ME, Gasbarrini A, Zocco MA. Mignini I, et al. Cells. 2023 Dec 31;13(1):90. doi: 10.3390/cells13010090. Cells. 2023. PMID: 38201294 Free PMC article. Review. - [6]-Gingerol Inhibits Chikungunya Virus Infection by Suppressing Viral Replication.
Hayati RF, Better CD, Denis D, Komarudin AG, Bowolaksono A, Yohan B, Sasmono RT. Hayati RF, et al. Biomed Res Int. 2021 Mar 27;2021:6623400. doi: 10.1155/2021/6623400. eCollection 2021. Biomed Res Int. 2021. PMID: 33855075 Free PMC article. - Recognition on pharmacodynamic ingredients of natural products.
Wang T, Fu ZY, Li YJ, Zi L, Song CZ, Tao YX, Zhang M, Gu W, Yu J, Yang XX. Wang T, et al. Saudi Pharm J. 2024 Jul;32(7):102124. doi: 10.1016/j.jsps.2024.102124. Epub 2024 Jun 2. Saudi Pharm J. 2024. PMID: 38933713 Free PMC article. Review. - Alternatives of mesenchymal stem cell-derived exosomes as potential therapeutic platforms.
Lee S, Jung SY, Yoo D, Go D, Park JY, Lee JM, Um W. Lee S, et al. Front Bioeng Biotechnol. 2024 Sep 9;12:1478517. doi: 10.3389/fbioe.2024.1478517. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39315312 Free PMC article. Review. - Grape exosome-like nanoparticles: A potential therapeutic strategy for vascular calcification.
Teng Y, He J, Zhong Q, Zhang Y, Lu Z, Guan T, Pan Y, Luo X, Feng W, Ou C. Teng Y, et al. Front Pharmacol. 2022 Oct 21;13:1025768. doi: 10.3389/fphar.2022.1025768. eCollection 2022. Front Pharmacol. 2022. PMID: 36339605 Free PMC article. Review.
References
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and Colon Cancer. Gastroenterology. 2010;138:2101–2114. - PubMed
- Stretch GL, Campbell BJ, Dwarakanath AD, Yaqoob M, Stevenson A, Morris AI, et al. 5-Amino salicylic acid absorption and metabolism in ulcerative colitis patients receiving maintenance sulphasalazine, olsalazine or mesalazine. Aliment Pharm Ther. 1996;10:941–7. - PubMed
- Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaili L, et al. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007;123:211–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical