aKMT Catalyzes Extensive Protein Lysine Methylation in the Hyperthermophilic Archaeon Sulfolobus islandicus but is Dispensable for the Growth of the Organism - PubMed (original) (raw)
. 2016 Sep;15(9):2908-23.
doi: 10.1074/mcp.M115.057778. Epub 2016 Jun 21.
Yanping Zhu 2, Yuling Chen 3, Wei Li 4, Zhenfeng Zhang 1, Di Liu 4, Tongkun Wang 1, Juncai Ma 5, Haiteng Deng 3, Zhi-Jie Liu 6, Songying Ouyang 7, Li Huang 8
Affiliations
- PMID: 27329856
- PMCID: PMC5013307
- DOI: 10.1074/mcp.M115.057778
aKMT Catalyzes Extensive Protein Lysine Methylation in the Hyperthermophilic Archaeon Sulfolobus islandicus but is Dispensable for the Growth of the Organism
Yindi Chu et al. Mol Cell Proteomics. 2016 Sep.
Abstract
Protein methylation is believed to occur extensively in creanarchaea. Recently, aKMT, a highly conserved crenarchaeal protein lysine methyltransferase, was identified and shown to exhibit broad substrate specificity in vitro Here, we have constructed an aKMT deletion mutant of the hyperthermophilic crenarchaeon Sulfolobus islandicus The mutant was viable but showed a moderately slower growth rate than the parental strain under non-optimal growth conditions. Consistent with the moderate effect of the lack of aKMT on the growth of the cell, expression of a small number of genes, which encode putative functions in substrate transportation, energy metabolism, transcriptional regulation, stress response proteins, etc, was differentially regulated by more than twofold in the mutant strain, as compared with that in the parental strain. Analysis of the methylation of total cellular protein by mass spectrometry revealed that methylated proteins accounted for ∼2/3 (1,158/1,751) and ∼1/3 (591/1,757) of the identified proteins in the parental and the mutant strains, respectively, indicating that there is extensive protein methylation in S. islandicus and that aKMT is a major protein methyltransferase in this organism. No significant sequence preference was detected at the sites of methylation by aKMT. Methylated lysine residues, when visible in the structure, are all located on the surface of the proteins. The crystal structure of aKMT in complex with S-adenosyl-l-methionine (SAM) or S-adenosyl homocysteine (SAH) reveals that the protein consists of four α helices and seven β sheets, lacking a substrate recognition domain found in PrmA, a bacterial homolog of aKMT, in agreement with the broad substrate specificity of aKMT. Our results suggest that aKMT may serve a role in maintaining the methylation status of cellular proteins required for the efficient growth of the organism under certain non-optimal conditions.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Fig. 1.
Growth curves of the parental, the mutant and the complementary strains. The three strains were grown at 65 °C (A and B) or 75 °C (C and D) in CVY-rich medium (A and C) or SCVy medium (B and D). The OD600 values of the cultures were measured. All numbers are an average of three independent measurements.
Fig. 2.
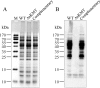
Detection of lysine methylation in total cellular proteins from the parental, the mutant and the complementary strains. Samples containing the same numbers of cells from each strain were loaded in parallel onto two 15% SDS-polyacrylamide gels. After electrophoresis, the two gels were subjected to staining with Commassie brilliant blue R-250 (A) and immunoblotting with anti-mono/dimethyllysine antibodies (B), respectively.
Fig. 3.
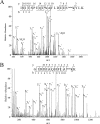
Representative MS/MS spectra of methylated peptides derived from trypsin-digested thermosome (SiRe_1214). The amino acid sequences of the peptides are shown with methylated lysine residues labeled. Mono, monomethylation, di, dimethylation.
Fig. 4.
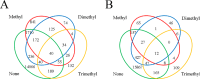
Schematic representation of lysine residues that were methylated to various extents. The extents of methylation on lysine residues, as identified by mass spectrometry in parent (A) or ΔaKMT (B), are depicted in a Venn diagram. Unmethylated, monomethylated, dimethylated or trimethylated lysine residues are indicated by none, methyl, dimethyl, or trimethyl, respectively.
Fig. 5.
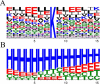
Sequence analysis of sites of methylation by aKMT. A, Primary structure. Different amino acid residues are shown in different colors. B, Predicted secondary structure. The secondary structures of the amino acid sequences containing the sites of methylation were predicted. Helix, sheet, turn and coil are represented by H, E, T and C, respectively. The frequencies of amino acid residues or secondary structure elements are generated by Weblogo.
Fig. 6.
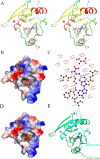
The crystal structures of aKMT-SAM and aKMT-SAH. A, Stereo view of the the aKMT-SAM structure. The structure is shown as a ribbon diagram with the α helices and the β sheets colored in red and yellow, respectively. The SAM molecule is shown as gray sticks. The purple sphere represents a magnesium ion. The N- and C termini are labeled with the respective letters. B, The solvent-accessible surface of aKMT in complex with SAM, colored according to electrostatic potential. Blue, positively charged; red, negatively charged; white, neutral. Electron density of a 2Fo-Fc simulated annealing (SA) omit map for SAM bound in the catalytic pocket contoured at 1.0σ is shown. The SAM molecule is shown as gray sticks. C, Schematic diagram summarizing the interactions between aKMT and SAM in the aKMT-SAM structure generated by LIGPLOT (67). Interacting atoms are connected by green dashed lines with bonding lengths indicated (in Å). Nonligand residues involved in direct hydrophobic contacts with SAM are shown as red semicircles with radiating spokes. D, The solvent-accessible surface of aKMT in complex with SAH, colored according to electrostatic potential. Blue, positively charged; red, negatively charged; white, neutral. The SAH molecule is shown as green sticks. E, Comparison of the structures of aKMT-SAH (cylan) and aKMT-SAM (green).
Fig. 7.
Thermal stability of total cellular proteins from the parental and the mutant strains. Cells from an exponentially grown culture of the parental strain or ΔaKMT were harvested, resuspended in 50 m
m
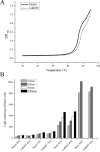
sodium phosphate buffer, pH 7.0, and sonicated. A, The clarified cell-free extracts were heated from 50 to 95 °C at a rate of 0.2 °C/min, and the increase in absorbance at 600 nm was recorded on a Shimadzu UV-2550 spectrophotometer. B, The cell-free extracts were incubated at 65, 75, 85, or 95 °C for 15, 30, 60, and 120 min. Protein aggregation was monitored by 90° light scattering at 488 nm on a Shimadzu RF5301PC spectrofluorimeter.
Fig. 8.
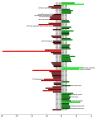
A bar plot showing genes differentially expressed in the parental and the mutant strains. Transcriptional profiles for the parental strain and ΔaKMT were determined and compared. Genes whose expression differed by more than twofold (FDR < 0.001) in the two strains, are shown. Red and green bars indicate down- and up-regulated genes, respectively. Putative functions of proteins discussed in the text are indicated.
Similar articles
- Identification and characterization of a highly conserved crenarchaeal protein lysine methyltransferase with broad substrate specificity.
Chu Y, Zhang Z, Wang Q, Luo Y, Huang L. Chu Y, et al. J Bacteriol. 2012 Dec;194(24):6917-26. doi: 10.1128/JB.01535-12. Epub 2012 Oct 19. J Bacteriol. 2012. PMID: 23086207 Free PMC article. - Abundant Lysine Methylation and N-Terminal Acetylation in Sulfolobus islandicus Revealed by Bottom-Up and Top-Down Proteomics.
Vorontsov EA, Rensen E, Prangishvili D, Krupovic M, Chamot-Rooke J. Vorontsov EA, et al. Mol Cell Proteomics. 2016 Nov;15(11):3388-3404. doi: 10.1074/mcp.M116.058073. Epub 2016 Aug 23. Mol Cell Proteomics. 2016. PMID: 27555370 Free PMC article. - Functional Insights Into Protein Acetylation in the Hyperthermophilic Archaeon Sulfolobus islandicus.
Cao J, Wang T, Wang Q, Zheng X, Huang L. Cao J, et al. Mol Cell Proteomics. 2019 Aug;18(8):1572-1587. doi: 10.1074/mcp.RA119.001312. Epub 2019 Jun 9. Mol Cell Proteomics. 2019. PMID: 31182439 Free PMC article. - Genetic manipulation in Sulfolobus islandicus and functional analysis of DNA repair genes.
Zhang C, Tian B, Li S, Ao X, Dalgaard K, Gökce S, Liang Y, She Q. Zhang C, et al. Biochem Soc Trans. 2013 Feb 1;41(1):405-10. doi: 10.1042/BST20120285. Biochem Soc Trans. 2013. PMID: 23356319 Review. - Genetic analyses in the hyperthermophilic archaeon Sulfolobus islandicus.
She Q, Zhang C, Deng L, Peng N, Chen Z, Liang YX. She Q, et al. Biochem Soc Trans. 2009 Feb;37(Pt 1):92-6. doi: 10.1042/BST0370092. Biochem Soc Trans. 2009. PMID: 19143609 Review.
Cited by
- The extraordinary thermal stability of EstA from S. islandicus is independent of post translational modifications.
Stiefler-Jensen D, Schwarz-Linnet T, de Lichtenberg C, Nguyen TTTN, Rand KD, Huang L, She Q, Teilum K. Stiefler-Jensen D, et al. Protein Sci. 2017 Sep;26(9):1819-1827. doi: 10.1002/pro.3220. Epub 2017 Jul 13. Protein Sci. 2017. PMID: 28681456 Free PMC article. - Pathogenic Leptospires Modulate Protein Expression and Post-translational Modifications in Response to Mammalian Host Signals.
Nally JE, Grassmann AA, Planchon S, Sergeant K, Renaut J, Seshu J, McBride AJ, Caimano MJ. Nally JE, et al. Front Cell Infect Microbiol. 2017 Aug 9;7:362. doi: 10.3389/fcimb.2017.00362. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28848720 Free PMC article. - Viruses of archaea: Structural, functional, environmental and evolutionary genomics.
Krupovic M, Cvirkaite-Krupovic V, Iranzo J, Prangishvili D, Koonin EV. Krupovic M, et al. Virus Res. 2018 Jan 15;244:181-193. doi: 10.1016/j.virusres.2017.11.025. Epub 2017 Nov 22. Virus Res. 2018. PMID: 29175107 Free PMC article. Review. - Post-Translational Modifications Aid Archaeal Survival.
Gong P, Lei P, Wang S, Zeng A, Lou H. Gong P, et al. Biomolecules. 2020 Apr 10;10(4):584. doi: 10.3390/biom10040584. Biomolecules. 2020. PMID: 32290118 Free PMC article. Review. - Lysine Methylation Modulates the Interaction of Archaeal Chromatin Protein Cren7 With DNA.
Ding N, Chen Y, Chu Y, Zhong C, Huang L, Zhang Z. Ding N, et al. Front Microbiol. 2022 Mar 3;13:837737. doi: 10.3389/fmicb.2022.837737. eCollection 2022. Front Microbiol. 2022. PMID: 35308404 Free PMC article.
References
- Lachner M., and Jenuwein T. (2002) The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 14, 286–298 - PubMed
- Paik W. K., Paik D. C., and Kim S. (2007) Historical review: the field of protein methylation. Trends Biochem. Sci. 32, 146–152 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources