ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies - PubMed (original) (raw)
. 2016 Aug 1;176(8):1155-66.
doi: 10.1001/jamainternmed.2016.2925.
Fumiaki Imamura 2, Stella Aslibekyan 3, Matti Marklund 4, Jyrki K Virtanen 5, Maria Wennberg 6, Mohammad Y Yakoob 1, Stephanie E Chiuve 7, Luicito Dela Cruz 8, Alexis C Frazier-Wood 9, Amanda M Fretts 10, Eliseo Guallar 11, Chisa Matsumoto 12, Kiesha Prem 13, Tosh Tanaka 14, Jason H Y Wu 15, Xia Zhou 16, Catherine Helmer 17, Erik Ingelsson 18, Jian-Min Yuan 19, Pascale Barberger-Gateau 17, Hannia Campos 20, Paulo H M Chaves 21, Luc Djoussé 22, Graham G Giles 8, Jose Gómez-Aracena 23, Allison M Hodge 8, Frank B Hu 24, Jan-Håkan Jansson 6, Ingegerd Johansson 25, Kay-Tee Khaw 26, Woon-Puay Koh 27, Rozenn N Lemaitre 28, Lars Lind 29, Robert N Luben 26, Eric B Rimm 24, Ulf Risérus 4, Cecilia Samieri 17, Paul W Franks 30, David S Siscovick 31, Meir Stampfer 24, Lyn M Steffen 16, Brian T Steffen 16, Michael Y Tsai 32, Rob M van Dam 33, Sari Voutilainen 5, Walter C Willett 24, Mark Woodward 34, Dariush Mozaffarian 35; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe)
Affiliations
- PMID: 27357102
- PMCID: PMC5183535
- DOI: 10.1001/jamainternmed.2016.2925
ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies
Liana C Del Gobbo et al. JAMA Intern Med. 2016.
Erratum in
- Explanation of Errors in Abstract and Supplement and in the Reporting of Analyses.
Mozaffarian D. Mozaffarian D. JAMA Intern Med. 2016 Nov 1;176(11):1727-1728. doi: 10.1001/jamainternmed.2016.6508. JAMA Intern Med. 2016. PMID: 27695819 No abstract available. - Errors in Abstract and Supplement and in Reporting of Analyses.
[No authors listed] [No authors listed] JAMA Intern Med. 2016 Nov 1;176(11):1728. doi: 10.1001/jamainternmed.2016.6514. JAMA Intern Med. 2016. PMID: 27695848 No abstract available. - Error in Author Affiliation.
[No authors listed] [No authors listed] JAMA Intern Med. 2019 Mar 1;179(3):457. doi: 10.1001/jamainternmed.2019.0135. JAMA Intern Med. 2019. PMID: 30742209 No abstract available.
Abstract
Importance: The role of ω-3 polyunsaturated fatty acids for primary prevention of coronary heart disease (CHD) remains controversial. Most prior longitudinal studies evaluated self-reported consumption rather than biomarkers.
Objective: To evaluate biomarkers of seafood-derived eicosapentaenoic acid (EPA; 20:5ω-3), docosapentaenoic acid (DPA; 22:5ω-3), and docosahexaenoic acid (DHA; 22:6ω-3) and plant-derived α-linolenic acid (ALA; 18:3ω-3) for incident CHD.
Data sources: A global consortium of 19 studies identified by November 2014.
Study selection: Available prospective (cohort, nested case-control) or retrospective studies with circulating or tissue ω-3 biomarkers and ascertained CHD.
Data extraction and synthesis: Each study conducted standardized, individual-level analysis using harmonized models, exposures, outcomes, and covariates. Findings were centrally pooled using random-effects meta-analysis. Heterogeneity was examined by age, sex, race, diabetes, statins, aspirin, ω-6 levels, and FADS desaturase genes.
Main outcomes and measures: Incident total CHD, fatal CHD, and nonfatal myocardial infarction (MI).
Results: The 19 studies comprised 16 countries, 45 637 unique individuals, and 7973 total CHD, 2781 fatal CHD, and 7157 nonfatal MI events, with ω-3 measures in total plasma, phospholipids, cholesterol esters, and adipose tissue. Median age at baseline was 59 years (range, 18-97 years), and 28 660 (62.8%) were male. In continuous (per 1-SD increase) multivariable-adjusted analyses, the ω-3 biomarkers ALA, DPA, and DHA were associated with a lower risk of fatal CHD, with relative risks (RRs) of 0.91 (95% CI, 0.84-0.98) for ALA, 0.90 (95% CI, 0.85-0.96) for DPA, and 0.90 (95% CI, 0.84-0.96) for DHA. Although DPA was associated with a lower risk of total CHD (RR, 0.94; 95% CI, 0.90-0.99), ALA (RR, 1.00; 95% CI, 0.95-1.05), EPA (RR, 0.94; 95% CI, 0.87-1.02), and DHA (RR, 0.95; 95% CI, 0.91-1.00) were not. Significant associations with nonfatal MI were not evident. Associations appeared generally stronger in phospholipids and total plasma. Restricted cubic splines did not identify evidence of nonlinearity in dose responses.
Conclusions and relevance: On the basis of available studies of free-living populations globally, biomarker concentrations of seafood and plant-derived ω-3 fatty acids are associated with a modestly lower incidence of fatal CHD.
Conflict of interest statement
Disclosures: Dr Barberger-Gateau reported receiving grants and nonfinancial support from Danone Research, Vifor Pharma, and Groupe Lipides et Nutrition; personal fees and nonfinancial support from Nutricia, ILSI Europe, and PiLeJe; and a grant from the Centre National Interprofessionnel de l’Industrie Laitière. Dr Del Gobbo reported receiving ad hoc consulting fees from the Life Sciences Research Organization. Dr Djoussé reported receiving investigator-initiated grants from Merck, Amarin Pharma Inc, California Walnut Commission, and the National Institutes of Health and serving as ad hoc consultant for Amarin Pharma Inc. Dr Helmer reported receiving fees for a conference from Novartis. Dr Mozaffarian reported receiving ad hoc honoraria from Bunge, Pollock Institute, and Quaker Oats; ad hoc consulting for Foodminds, Life Sciences Research Organization, Nutrition Impact, Amarin, AstraZeneca, Winston, and Strawn LLP; membership in Unilever North America Scientific Advisory Board; and chapter royalties from UpToDate. Dr Woodward reported receiving consulting fees from Amgen and Novartis and research funding from Sanofi. No other disclosures were reported.
Figures
Figure 1
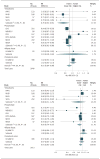
Relative Risk (RR) of Fatal Coronary Heart Disease (CHD) per 1-SD Increase in the Biomarkers α-Linolenic Acid (ALA; 18:3ω-3) and Eicosapentaenoic Acid (EPA; 20:5ω-3) Estimates were pooled using random effects meta-analysis. See eFigure 1 in the Supplement for results using inverse-variance weights. CHS indicates Cardiovascular Health Study; EPIC-Norfolk, European Prospective Investigation of Cancer (Norfolk); HPFS, Health Professionals Follow-up Study; KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study; MCCS, Melbourne Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; NHS, Nurses’ Health Study; NSHDS, Northern Sweden Health and Disease Study,; PHS, Physician’s Health Study; SCHS, Singapore Chinese Health Study; and ULSAM, Uppsala Longitudinal Study of Adult Men.
Figure 2
Relative Risk (RR) of Fatal Coronary Heart Disease (CHD) per 1-SD Increase in the Biomarkers Docosapentaenoic Acid (DPA; 22:5ω-3) and Docosahexaenoic Acid (DHA; 22:6ω-3) Estimates were pooled using random effects meta-analysis. See eFigure 1 in the Supplement for results using inverse-variance weights. CHS indicates Cardiovascular Health Study; EPIC-Norfolk, European Prospective Investigation of Cancer (Norfolk); HPFS, Health Professionals Follow-up Study; KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study; MCCS, Melbourne Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; NHS, Nurses’ Health Study; NSHDS, Northern Sweden Health and Disease Study,; PHS, Physician’s Health Study; SCHS, Singapore Chinese Health Study; SHHEC, Scottish Heart Health Extended Chart; and ULSAM, Uppsala Longitudinal Study of Adult Men.
Comment in
- Long-Chain Omega-3 Fatty Acid Bioavailability: Implications for Understanding the Effects of Supplementation on Heart Disease Risk.
Maki KC. Maki KC. J Nutr. 2018 Nov 1;148(11):1701-1703. doi: 10.1093/jn/nxy205. J Nutr. 2018. PMID: 30383282 No abstract available.
Similar articles
- Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study.
Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Mozaffarian D, et al. Ann Intern Med. 2013 Apr 2;158(7):515-25. doi: 10.7326/0003-4819-158-7-201304020-00003. Ann Intern Med. 2013. PMID: 23546563 Free PMC article. - Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality.
Jiang H, Wang L, Wang D, Yan N, Li C, Wu M, Wang F, Mi B, Chen F, Jia W, Liu X, Lv J, Liu Y, Lin J, Ma L. Jiang H, et al. Clin Nutr. 2022 Aug;41(8):1798-1807. doi: 10.1016/j.clnu.2022.06.034. Epub 2022 Jun 30. Clin Nutr. 2022. PMID: 35830775 - n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study.
Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. Lemaitre RN, et al. Am J Clin Nutr. 2003 Feb;77(2):319-25. doi: 10.1093/ajcn/77.2.319. Am J Clin Nutr. 2003. PMID: 12540389 - Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease.
Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, AlAbdulghafoor FK, Summerbell CD, Worthington HV, Song F, Hooper L. Abdelhamid AS, et al. Cochrane Database Syst Rev. 2018 Nov 30;11(11):CD003177. doi: 10.1002/14651858.CD003177.pub4. Cochrane Database Syst Rev. 2018. PMID: 30521670 Free PMC article. Updated. - Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review.
Balk EM, Adams GP, Langberg V, Halladay C, Chung M, Lin L, Robertson S, Yip A, Steele D, Smith BT, Lau J, Lichtenstein AH, Trikalinos TA. Balk EM, et al. Evid Rep Technol Assess (Full Rep). 2016 Aug;(223):1-1252. doi: 10.23970/AHRQEPCERTA223. Evid Rep Technol Assess (Full Rep). 2016. PMID: 30307737
Cited by
- Impact of α-Linolenic Acid, the Vegetable ω-3 Fatty Acid, on Cardiovascular Disease and Cognition.
Sala-Vila A, Fleming J, Kris-Etherton P, Ros E. Sala-Vila A, et al. Adv Nutr. 2022 Oct 2;13(5):1584-1602. doi: 10.1093/advances/nmac016. Adv Nutr. 2022. PMID: 35170723 Free PMC article. - Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved.
Watanabe Y, Tatsuno I. Watanabe Y, et al. J Atheroscler Thromb. 2020 Mar 1;27(3):183-198. doi: 10.5551/jat.50658. Epub 2019 Oct 3. J Atheroscler Thromb. 2020. PMID: 31582621 Free PMC article. Review. - Recent Clinical Trials Shed New Light on the Cardiovascular Benefits of Omega-3 Fatty Acids.
Kris-Etherton PM, Richter CK, Bowen KJ, Skulas-Ray AC, Jackson KH, Petersen KS, Harris WS. Kris-Etherton PM, et al. Methodist Debakey Cardiovasc J. 2019 Jul-Sep;15(3):171-178. doi: 10.14797/mdcj-15-3-171. Methodist Debakey Cardiovasc J. 2019. PMID: 31687095 Free PMC article. Review. - Comparison of dimension reduction methods on fatty acids food source study.
Chen Y, Miura Y, Sakurai T, Chen Z, Shrestha R, Kato S, Okada E, Ukawa S, Nakagawa T, Nakamura K, Tamakoshi A, Chiba H, Imai H, Minami H, Mizuta M, Hui SP. Chen Y, et al. Sci Rep. 2021 Sep 21;11(1):18748. doi: 10.1038/s41598-021-97349-6. Sci Rep. 2021. PMID: 34548525 Free PMC article. - The VITamin D and OmegA-3 TriaL (VITAL): Do Results Differ by Sex or Race/Ethnicity?
Bassuk SS, Chandler PD, Buring JE, Manson JE; VITAL Research Group. Bassuk SS, et al. Am J Lifestyle Med. 2020 Dec 24;15(4):372-391. doi: 10.1177/1559827620972035. eCollection 2021 Jul-Aug. Am J Lifestyle Med. 2020. PMID: 34366734 Free PMC article. Review.
References
- Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. - PubMed
- Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112(4):298–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HC095161/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- R01 HL059367/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- UM1 CA167552/CA/NCI NIH HHS/United States
- R01 CA097193/CA/NCI NIH HHS/United States
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- R21 HL088081/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- R01 HL085710/HL/NHLBI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- Z99 AG999999/ImNIH/Intramural NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- MR/N003284/1/MRC_/Medical Research Council/United Kingdom
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N02 HL064278/HL/NHLBI NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- BHF_/British Heart Foundation/United Kingdom
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- R01 MD009164/MD/NIMHD NIH HHS/United States
- P01 CA055075/CA/NCI NIH HHS/United States
- HHSN268200800007C/HL/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- R01 HL034595/HL/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- R01 CA144034/CA/NCI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- G1000143/MRC_/Medical Research Council/United Kingdom
- N01 HC095167/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- G0401527/MRC_/Medical Research Council/United Kingdom
- R01 HL034594/HL/NHLBI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- R01 HL026490/HL/NHLBI NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- R01 HL035464/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- R01 HL088521/HL/NHLBI NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- R01 HL060712/HL/NHLBI NIH HHS/United States
- R01 AA011181/AA/NIAAA NIH HHS/United States
- R01 CA049449/CA/NCI NIH HHS/United States
- R01 CA040360/CA/NCI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- CSO_/Chief Scientist Office/United Kingdom
- R01 AG023629/AG/NIA NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
- R01 HL081549/HL/NHLBI NIH HHS/United States
- R01 CA034944/CA/NCI NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- UM1 CA182876/CA/NCI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials