Novel perspectives on therapeutic modulation of the gut microbiota - PubMed (original) (raw)
Review
Novel perspectives on therapeutic modulation of the gut microbiota
Justin L McCarville et al. Therap Adv Gastroenterol. 2016 Jul.
Abstract
The gut microbiota contributes to the maintenance of health and, when disrupted, may drive gastrointestinal and extragastrointestinal disease. This can occur through direct pathways such as interaction with the epithelial barrier and mucosal immune system or indirectly via production of metabolites. There is no current curative therapy for chronic inflammatory conditions such as inflammatory bowel disease, which are complex multifactorial disorders involving genetic predisposition, and environmental triggers. Therapies are directed to suppress inflammation rather than the driver, and these approaches are not devoid of adverse effects. Therefore, there is great interest in modulation of the gut microbiota to provide protection from disease. Interventions that modulate the microbiota include diet, probiotics and more recently the emergence of experimental therapies such as fecal microbiota transplant or phage therapy. Emerging data indicate that certain bacteria can induce protective immune responses and enhance intestinal barrier function, which could be potential therapeutic targets. However, mechanistic links and specific therapeutic recommendations are still lacking. Here we provide a pathophysiological overview of potential therapeutic applications of the gut microbiota.
Keywords: fecal microbiota transplant; inflammatory bowel disease; irritable bowel syndrome; microbiota; prebiotics; probiotics.
Conflict of interest statement
Conflict of interest statement: EFV receives funding from Nestle.
Figures
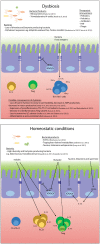
Figure 1.
Proposed homeostatic and dysbiotic mechanisms of the microbiota and possible therapeutic interventions. AMP, antimicrobial peptides; FMT, fecal microbiota transplant; HDAC, histone deacetylase; IFN, interferon; IL, interleukin; ILC, innate lymphoid cell; NLRP6, NOD-like receptor family pyrin domain containing 6; SCFA, short chain fatty acid; Treg, T-regulatory cell.
Similar articles
- Modulation of the gut microbiota: a focus on treatments for irritable bowel syndrome.
Harris LA, Baffy N. Harris LA, et al. Postgrad Med. 2017 Nov;129(8):872-888. doi: 10.1080/00325481.2017.1383819. Epub 2017 Oct 13. Postgrad Med. 2017. PMID: 28936910 Review. - The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy.
Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. Cammarota G, et al. Pharmacol Ther. 2015 May;149:191-212. doi: 10.1016/j.pharmthera.2014.12.006. Epub 2015 Jan 3. Pharmacol Ther. 2015. PMID: 25561343 Review. - Gut microbiota role in irritable bowel syndrome: New therapeutic strategies.
Distrutti E, Monaldi L, Ricci P, Fiorucci S. Distrutti E, et al. World J Gastroenterol. 2016 Feb 21;22(7):2219-41. doi: 10.3748/wjg.v22.i7.2219. World J Gastroenterol. 2016. PMID: 26900286 Free PMC article. Review. - Gut Microbiota and Chronic Constipation: A Review and Update.
Ohkusa T, Koido S, Nishikawa Y, Sato N. Ohkusa T, et al. Front Med (Lausanne). 2019 Feb 12;6:19. doi: 10.3389/fmed.2019.00019. eCollection 2019. Front Med (Lausanne). 2019. PMID: 30809523 Free PMC article. Review. - The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies.
West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL; in-FLAME Microbiome Interest Group. West CE, et al. J Allergy Clin Immunol. 2015 Jan;135(1):3-13; quiz 14. doi: 10.1016/j.jaci.2014.11.012. J Allergy Clin Immunol. 2015. PMID: 25567038 Review.
Cited by
- Pseudomonas aeruginosa Bacteriophages and Their Clinical Applications.
Alipour-Khezri E, Skurnik M, Zarrini G. Alipour-Khezri E, et al. Viruses. 2024 Jun 29;16(7):1051. doi: 10.3390/v16071051. Viruses. 2024. PMID: 39066214 Free PMC article. Review. - Unlocking the gut-heart axis: exploring the role of gut microbiota in cardiovascular health and disease.
Shariff S, Kwan Su Huey A, Parag Soni N, Yahia A, Hammoud D, Nazir A, Uwishema O, Wojtara M. Shariff S, et al. Ann Med Surg (Lond). 2024 Jan 18;86(5):2752-2758. doi: 10.1097/MS9.0000000000001744. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694298 Free PMC article. Review. - Targeted Antimicrobial Agents as Potential Tools for Modulating the Gut Microbiome.
Chou S, Zhang S, Guo H, Chang YF, Zhao W, Mou X. Chou S, et al. Front Microbiol. 2022 Jul 7;13:879207. doi: 10.3389/fmicb.2022.879207. eCollection 2022. Front Microbiol. 2022. PMID: 35875544 Free PMC article. Review. - Any Future for Faecal Microbiota Transplantation as a Novel Strategy for Gut Microbiota Modulation in Human and Veterinary Medicine?
Takáčová M, Bomba A, Tóthová C, Micháľová A, Turňa H. Takáčová M, et al. Life (Basel). 2022 May 12;12(5):723. doi: 10.3390/life12050723. Life (Basel). 2022. PMID: 35629390 Free PMC article. Review. - Prevotella histicola Protects From Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice.
Balakrishnan B, Luckey D, Bodhke R, Chen J, Marietta E, Jeraldo P, Murray J, Taneja V. Balakrishnan B, et al. Front Immunol. 2021 May 4;12:609644. doi: 10.3389/fimmu.2021.609644. eCollection 2021. Front Immunol. 2021. PMID: 34017324 Free PMC article.
References
- Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., et al. (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500: 232–236. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources