An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport - PubMed (original) (raw)
An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport
Marlieke L M Jongsma et al. Cell. 2016.
Abstract
Through a network of progressively maturing vesicles, the endosomal system connects the cell's interior with extracellular space. Intriguingly, this network exhibits a bilateral architecture, comprised of a relatively immobile perinuclear vesicle "cloud" and a highly dynamic peripheral contingent. How this spatiotemporal organization is achieved and what function(s) it curates is unclear. Here, we reveal the endoplasmic reticulum (ER)-located ubiquitin ligase Ring finger protein 26 (RNF26) as the global architect of the entire endosomal system, including the trans-Golgi network (TGN). To specify perinuclear vesicle coordinates, catalytically competent RNF26 recruits and ubiquitinates the scaffold p62/sequestosome 1 (p62/SQSTM1), in turn attracting ubiquitin-binding domains (UBDs) of various vesicle adaptors. Consequently, RNF26 restrains fast transport of diverse vesicles through a common molecular mechanism operating at the ER membrane, until the deubiquitinating enzyme USP15 opposes RNF26 activity to allow vesicle release into the cell's periphery. By drawing the endosomal system's architecture, RNF26 orchestrates endosomal maturation and trafficking of cargoes, including signaling receptors, in space and time.
Keywords: E3 ligase; EGFR signaling; EPS15; ER; RNF26; TAX1BP1; TGN; TOLLIP; USP15; endosomes; lysosomes; perinuclear; transport; ubiquitin.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
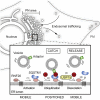
Graphical abstract
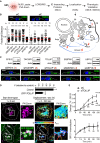
Figure 1
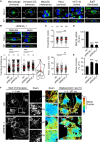
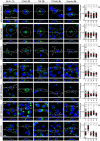
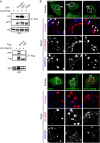
RNF26 Depletion Disrupts Spatiotemporal Organization of Endosomes (A) Intracellular distribution of LEs (CD63, green) in various cell lines. Representative maximum z projection (3D) overlays with nuclear DAPI (blue) and their corresponding z cross sections along the demarcated line are shown. Cell boundaries are depicted in dashed lines. For other markers, see Figure S1. (B) Effect of RNF26 depletion on distribution of LEs, represented as fractional distances of CD63 vesicles from center of nucleus (distance of pixels from nucleus = faction of distance from nucleus to the plasma membrane [1.0]; mean shown in red). For 3D view, see Movies S1A and S1B. (C) Cell shape analysis for samples in (B), showing total cell area and eccentricity calculated in an automated fashion as described in the Supplemental Experimental Procedures. (D) mRNA levels of RNF26 targeted by two different siRNAs (siRNF26_1 and siRNF26_2) as assessed by qPCR are expressed relative to siC; n = 3. (E) Quantification of the mobile fraction of acidified Lysotracker (LT)-positive vesicles (LTVs) as a function of RNF26; n = 3. For details, refer to the Supplemental Experimental Procedures. (F) Organization and dynamics of LTVs (white) in control (siC) versus RNF26-depleted (siRNF26_1) MelJuSo cells. Left panels: representative single confocal plane fluorescence images taken at the start of time lapse. Right panels: vesicle displacement rates (blue, immobile; red, max mobility) observed over the 297-s time interval. Nuclei and cell boundaries are depicted in dashed lines, and zoom-ins highlight peripheral (PP) and perinuclear (PN) boxed regions. Quantification appears in (E). For LT time lapses, see Movies S2A–S2C. For CD63 time lapses see Movies S2D and S2E. Scale bars, 10 μm. For all figures: n, # of cells analyzed per condition; n, # independent experiments; error bars, SD.
Figure 2
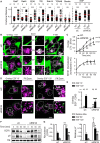
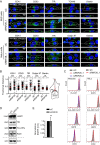
RNF26 Promotes Cargo Trafficking and Endosome Maturation in the Perinuclear Cloud (A) Intracellular distribution of various markers and cargoes in MelJuSo cells in response to RNF26 depletion, presented as fractional distances with mean shown in red, as in Figure 1B. Late endosomes, Rab7; early endosomes, EEA1, Rab5, and Rab14; recycling endosomes, Transferrin receptor (TfR); TGN, TGN46; Golgi, Giantin; ligand-mediated endocytosis, EGF. See also Figures S2A, S2B, and 2D and Movies S3A–S3D. (B) Trafficking of fluid phase dye SR101 (green) to the acidified compartment (LT, magenta). Single confocal plane fluorescence overlays with PP and PN zooms at t = 0 and t = 150 min following addition of SR101 to control or RNF26-depleted MelJuSo cells are shown. See also Movies S4A and S4B. (C) Top graph: quantification (Mander’s overlap) of SR101 entry into LTVs as a function of time (min) in control MelJuSo cells or those silenced for RNF26. n = 2. Bottom graph: total uptake of SR101 in control or RNF26-depleted MelJuSo cells as measured by flow cytometry, expressed as fold increase normalized to t = 0 as a function of time; n = 3. (D) Effect of RNF26 depletion on receptor-mediated trafficking of EGF-555 (green) to the late endosome compartment (CD63, magenta). Representative z projection (3D) overlays at 10 min (left panels) and 120 min (right panels) following stimulation (100 ng/ml) are shown with PN zooms for control and RNF26-depleted HeLa cells. (E) Colocalization (Mander’s overlap) of EGF with CD63 at 10 min (white) and 120 min (black) following ligand stimulation; n = 2. (F) EGFR degradation in control versus RNF26-depleted HeLa cells. Immunoblots against total (EGFR) and phosphorylated (pY) EGFR, as well as TfR (loading control) along a time course following 20 ng/ml EGF addition (min) are shown (lane corresponding to t = 10 min was excised from siC panel). For analysis of surface and total EGFR levels see Figures S2C and S2E, respectively. (G) Quantification of total (left graph, relative to t = 0) and activated (right graph, pY relative to siC at t = 30′) EGFR as a function of time following EGF addition; n = 3. Scale bars, 10 μm.
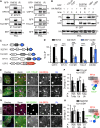
Figure 3
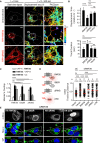
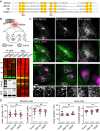
ER-Associated Ubiquitin Ligase Activity of RNF26 Organizes the PN Cloud and Controls Vesicle Dynamics (A) Effect of RNF26 on vesicle dynamics. Left panels: representative single confocal plane fluorescence overlays of LT (white) in control (untransfected; focal plane through the nucleus) HeLa cells or those ectopically expressing (red) RFP-RNF26 (focal plane just above the nucleus) or its ΔRING mutant (focal plane through the nucleus) at the start of time lapse are shown. Middle panels: corresponding vesicle displacement rates (blue, immobile; red, max mobility) observed during the 343-s time interval. Right panels: zooms of boxed PN regions. (B) Quantification of displacement rates (relative to untransfected cells) and track duration times (s) for data presented in (A); n = 4. (C) Colocalization (Mander’s overlap) of RNF26 or its mutants I382R and ΔRING with the ER protein VAP-A or ubiquitin. White bars, overlap VAP-A with RNF26; black bars, overlap RNF26 with VAP-A; gray bars, overlap RNF26 with ubiquitin; n = 10 cells per sample per experiment, n = 3. See also Figures S3A–S3C. (D) Rescue of RNF26 depletion phenotype (siRNF26 targeting 3′UTR) by re-expression of wild-type HA-RNF26, its RING domain mutants I382R and ΔRING, its _trans_-membrane truncation ΔTM or empty vector. Late endosome (LE) fractional distance analysis of CD63 in MelJuSo cells is shown (mean in red; number of data points analyzed per condition in parenthesis), along with a schematic overview of RNF26 constructs used. For cell shape analysis of ectopic RNF26 expression, see Figure S3D. (E) Selected representative maximum z projection (3D) image overlays of CD63 (green) with nuclear DAPI (blue) corresponding to quantification in (D) are shown, together with their z cross sections along the demarcated line. Single confocal slices in top panels show HA-tagged expression. Scale bars, 10 μm.
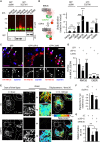
Figure 4
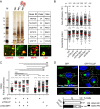
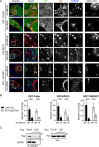
Protein Network Associated with the RING Domain of RNF26 (A) Workflow scheme for the identification and validation of proteins interacting with the cytosolic domain of RNF26. RING-associated RNF26 proteome consists of membrane-associated adaptor proteins and a DUB USP15 (for proteomic analysis details, see Figure S4A). Localization of RNF26-interacting proteins EPS15, TOLLIP, TAX1BP1, SQSTM1, and USP15 are depicted schematically (for representative marker overlays see Figure S4A). CCV, clathrin-coated vesicle; EE, early endosome; RE, recycling endosome; LE, late endosome; Ly, lysosome; TGN, _trans_-Golgi network; AUT, autophagosome; PM, plasma membrane. (B) Intracellular distribution (fractional distance analysis, mean shown in red) of LEs (CD63) and TGN (TGN46) in MelJuSo cells as a function of indicated siRNA perturbations. For cell shape analysis, see Figure S4B. For combinatorial silencing of vesicle adaptors, see Figure S4C. (C) Representative z-cross section (3D) image overlays of CD63 (green, upper panels) or TGN46 (green, bottom panels) with nuclear DAPI (blue) in MelJuSo cells are shown with the corresponding protein levels of silenced targets (left lanes) as compared to the control (right lanes). (D) Effect of GFP-TOLLIP (green) overexpression on the organization and dynamics of acidified vesicles (LTVs, magenta) in HeLa cells. Left panels: representative single confocal plane fluorescence image overlays taken at the start of the time lapse. Right panels: corresponding vesicle displacement rates (blue, immobile; red, max mobility) observed during the 343-s time interval; zoom-ins highlight boxed PN regions. Quantification of LTV dynamics (displacement/s relative to untransfected cells) as a function of TOLLIP is shown above the images; n = 2. See also Figure S4D and Movies S5A and S5B. (E) Top graph: quantification (Mander’s overlap) of SR101 entry into acidified vesicles (LTVs) as a function of time (min) in control MelJuSo cells (siC; control dataset in common with Figure 2C) versus those depleted of TOLLIP (siTOLLIP). n = 2. Bottom graph: total uptake of SR101 in control or TOLLIP-depleted MelJuSo cells as measured by flow cytometry, expressed as fold increase normalized to t = 0 as a function of time; n = 3. Scale bars, 10 μm.
Figure 5
RNF26 Couples to Ubiquitin-Binding Domains of Specific Membrane Adaptors (A) Interactions (assayed by coIP) between RNF26 versus its inactive mutant I382R (IR) and endogenous EPS15, TAX1BP1, TOLLIP, and USP15 in HEK293T cells. WCL, whole-cell lysate. (B) Effects of mutations in ubiquitin-binding domains (UBDs) of TOLLIP and SQSTM1 on interaction with RNF26 in HEK293T cells (extraneous lanes between 4 and 5, as well as 6 and 7 were excised). For TAX1BP1 and EPS15, see Figure S5A. (C) Schematic: domain organization of RNF26-interacting proteins, highlighting membrane-targeting domains (gray), UBDs (blue), and USP (red). (D) Quantification of interactions (normalized as % of WT/WT coIP, black bars) as a function of RNF26 inactivation (I382R white bars) or loss of UBD capabilities (UBA∗/ΔUBA, blue bars) for each pair of proteins; n = 3. (E–H) Colocalization of GFP-tagged (green) (E) TOLLIP (quantified in F; n = 2) and (G) SQSTM1 (quantified in H; n = 2) or their UBD mutants (CUE∗ and ΔUBA, respectively) with HA-RNF26 (red) and endogenous ubiquitin (blue) in MelJuSo cells. Representative single confocal plane fluorescence overlays and single-channel zooms are shown. Summary is illustrated schematically at the right. Scale bars, 10 μm.
Figure 6
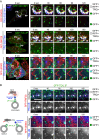
SQSTM1 Is a Substrate for RNF26 and the DUB USP15 (A) Ubiquitination status of SQSTM1 as a function of catalytic activities of RNF26 and USP15. GFP-SQSTM1 (or GFP-ΔUBA) was isolated from HEK293T cells overexpressing HA-Ub in the presence of vector, RFP-RNF26 versus its catalytic mutant I382R, or USP15 versus its catalytic mutant C269S. Ubiquitination status of GFP-substrate (green) was assessed by immunoblots against HA (red). (B) Quantification of the ubiquitination assay in (A); n = 4. Schematic on the left depicts proposed catalytic opposition between RNF26 and USP15. (C) Effect of USP15 on localization of SQSTM1 (blue) at RNF26-positive sites (red) in the PN area. Representative single confocal plane fluorescence overlays of PN regions are shown with their corresponding single channel images (HeLa cells). For a full image panel, see Figure S5B. Scale bar, 2.5 μm. (D) Quantification of RNF26 or I382R occupancy by SQSTM1 (Mander’s overlap) as a function of USP15 catalytic activity; n = 2. (E) Organization and dynamics of acidified LT-positive vesicles (LTVs, white) in control (siC) versus USP15-depleted (siUSP15) HeLa cells. Left panels: representative single confocal plane fluorescence images at the start of time lapse are shown. Right panels: corresponding vesicle displacement rates (blue, immobile; red, max mobility) observed during the 343-s time interval; zoom-ins highlight boxed PP and PN regions. For time lapses, see Movies S5A and S5C. Scale bar, 10 μm. (F) Effect of USP15 depletion on LTV dynamics (displacement/s relative to cells transfected with control siRNA); n = 2. (G) Functional interplay between siUSP15 and siRNF26. Quantification of mobile LTV fraction as a function of indicated siRNA perturbations (+) in MelJuSo cells; n = 2.
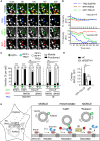
Figure 7
RNF26/SQSTM1 Complex Positions and Retains Adaptor-Selected Vesicles (A) Overlay zooms of frames selected from a time lapse (Movie S7) of vesicles marked by GFP-TOLLIP (green) in the presence of TRQ-SQSTM1 (blue) and RFP-RNF26 (red) in HeLa cells. Arrowheads point to two vesicles profiled in (B). Scale bar, 2.5 μm. (B) Plots of signal intensities over time corresponding to a positioned vesicle 1 (top graph) and a released vesicle 2 (bottom graph) as observed in (A). Dashed lines show background signal for each channel. (C) Quantification of adaptor-selected vesicle dynamics (mobile, white; positioned, black) expressed as % of vesicles per category (number counted given above each bar). GFP-marked vesicles (green); vesicles colocalizing with RFP-RNF26/-ΔRING and/or TRQ-SQSTM1 (white and cyan, respectively). See also Figures S6 and S7 and Movies S6A and S6B. (D) Co-localization (Mander’s overlap) between RNF26 and GFP-TOLLIP in control (siC) and SQSTM1-depleted (siSQSTM1) MelJuSo cells. (E) Model of vesicle positioning in the PN cloud by the RNF26 system. (1) Adaptor-selected (green) vesicles are subject to fast microtubule-based transport when unanchored by RNF26. (2) Catalytically competent RNF26 (light red) recruits SQSTM1 (blue) and mediates ubiquitin ligation (red), which serves to attract UBDs of specific vesicle-associated adaptors. On engagement, this multi-protein complex positions cognate vesicles (early, recycling, and late endosomes, and TGN) in the perinuclear space. (3) Dissociation of the RNF26/SQSTM1 complex, promoted by the DUB USP15 (yellow), releases target vesicles for (4) fast transport into the cell periphery.
Figure S1
A Wallpaper Showing Endosomal and Golgi Distribution in Various Cell Types, Related to Figure 1 Intracellular distribution of early endosomes (EEA1 (1), green), LEs/Lysosomes (CD63 (2), green), recycling endosomes (TfR (3), green), Trans-Golgi network (TGN46 (4), green), and Golgi (Giantin (5), green) in various cell lines (3 human primary immune cells, monocytes, macrophages, and immature dendritic cells, and 4 human cell lines including the primary BJET fibroblast cell line). Representative maximum z-projection (3D) overlays with nuclear DAPI (blue) and their corresponding z-cross sections along the demarcated line are shown below the X-Y images. Cell boundaries are depicted in dashed lines. Fractional distance analysis of marked vesicles in various cell lines are shown (right panels) reported as distance of pixels from nucleus = faction of distance from nucleus to the plasma membrane (max 1.0); mean shown in red. Numbers below the figure relate to the marker proteins indicated above the microscopy images; n = 2; scale bar, 10 μm; n = # of cells analyzed per condition, with # of data points analyzed per condition given in parenthesis; n, # independent experiments.
Figure S2
Effect of RNF26 Depletion on the Expression and Distribution of Various Marker Proteins, Related to Figure 2 (A) MelJuSo cells (top panels) and HeLa cells (bottom, panels) transfected with siRNA against RNF26 (siRNF26_1) or control siRNA (siC), as indicated. Intracellular distribution of Early endosomes (EEA1, green), LEs/Lysosomes (CD63, green), recycling endosomes (TfR, green), Trans-Golgi network (TGN46, green) and the Golgi (Giantin, green) are shown. Representative maximum z-projection (3D) overlays with nuclear DAPI (blue) and their corresponding z-cross sections along the demarcated line are shown below the X-Y images. Cell boundaries are depicted in dashed lines; n = 2. (B) Intracellular distribution of various markers and cargoes in HeLa cells in response to RNF26 depletion (siRNF26), shown in (A), presented as fractional distances with mean shown in red. For MelJuSo cells, see Figure 2A. (C) Cell surface expression of various markers in RNF26-depleted MelJuSo cells (left panels, MHC class II (HLA-DR), MHC class I (HLA-ABC), M6PR and CD63) or HeLa cells (right panels, EGFR, MHC class I (HLA-ABC), M6PR and CD63) analyzed by flow cytometry; n = 3. (D) Total levels of endosomal marker proteins and cargoes in MelJuSo cells depleted of RNF26 (siRNF26) compared to control cells (siC) analyzed by SDS-PAGE and WB using β-actin as loading control. Lysosomes (LAMP1); recycling endosomes (TfR); MIIC (MHC class II: HLA-DRa) and (late)-endosomes (Rab9). (E) Total cellular abundance of EGFR in HeLa cells depleted of RNF26 (siRNF26) compared to control cells (siC) analyzed by SDS-PAGE and WB using TfR as loading control; n = 3. Scale bar, 10 μm; n = # of cells analyzed per condition, n = # independent experiments, error bars = SD.
Figure S3
The RING Domain of RNF26 Mediates Its Localization and Function, Related to Figure 3 (A) Sequence comparison of the RNF26 RING domain with the RING domains of three other structurally related E3-ligases (BRCA1, CBL and TRIM13). The amino acids required for the Zn2+ positioning are indicated in yellow; amino acids mutated to inactivate RNF26 are indicated with ∗ (Iso 382 and Cys 401). (B) (top) Schematic overview of the RNF26 RING domain: residues Iso382 and Cys401 mutated in this study are indicated in red. (bottom) RFP-RNF26 and its inactive mutants, co-expressed with HA-Ubiquitin in HEK293 cells, were immunoprecipitated under harsh lysis conditions, separated by SDS-PAGE and analyzed by WB to visualize the associated modifications with HA-Ub (left panel). Whole-cell lysate (WCL) controls are shown on the right. (C) RFP-RNF26 or the indicated mutants (magenta) were expressed in MelJuSo cells and co-stained for VAP-A (green) to label the ER or with Ubiquitin (green). Zoom-in of the indicated region illustrates effective co-labeling of WT, but not catalytically incompetent RNF26 with Ub. (D) Effect of ectopical expression of RNF26 or its inactive mutant I382R on the size and eccentricity in either MelJuSo (left panels) or HeLa cells (right panels) (mean shown in red); n = 2. n = # of cells analyzed per condition, n = # independent experiments, error bars = SD; scale bar, 10 μm.
Figure S4
Isolation and Characterization of Proteins Associated to the RNF26 Cytoplasmic Domain, Related to Figure 4 (A) (left) Isolation and characterization of RNF26 associated proteome interacting with its RING domain. Two GST-tagged RNF26 tails (aa304-433 and aa363-433) including the RING domain were used (last two lanes) and compared to GST-tag only as a control (first lane). The Silver-stained gel (including marker lane) with bands isolated and identified by mass spectrometry marked as indicated. (right) Table showing the number of unique identified peptides (#pts) corresponding to RNF26 and its interacting partners TAX1BP1, SQSTM1, EPS15, TOLLIP and USP15 that were absent in the GST-only control lane. (bottom) Zoom-in of identified endogenous proteins along with marker proteins for their vesicular localization in MelJuSo cells. EPS15 (green) marks clathrin-coated vesicles (clathrin, red); TOLLIP (green) locates to late endosomes (CD63, red); TAX1BP1 partially co-localizes with secreted M6PR (red) and SQSTM1 locates to autophagosomes (LC3, red). (B) Effect of protein depletion (identified in (A) on cell size and shape (eccentricity) in MelJuSo cells compared to control cells (siC) with mean shown in red; n = 2. (C) Fractional distance analysis of CD63 marked late endosomes in MelJuSo cells after silencing one or multiple ubiquitin adaptors identified. Mean in red and statistical significance relative to the control (siC) are indicated. (D) In contrast to other cell types analyzed, RKO tumor cell line displayed unusual distribution of CD63 (green, upper panel, see also Figure S1). Overexpression of GFP-TOLLIP in these cells re-clusters CD63 positive vesicles (green, bottom panel). Representative maximum z-projection (3D) overlays with nuclear DAPI (blue) and their corresponding z-cross sections along the demarcated line are shown below the X-Y images. Cell boundaries are depicted in dashed lines. GFP signal was omitted from the presented image, but use to define the transfected cells. Quantification of perinuclear (PN) and peripheral (PP) positioning of CD63 positive vesicles following GFP or GFP-TOLLIP expression; n = 2. Scale bar, 10 μm; n = # of cells analyzed per condition, n = # independent experiments, error bars = SD.
Figure S5
RNF26 Interacts with Ubiquitin-Binding Domains of Vesicle Adaptors, and USP15 Reduces Co-localization of RNF26 and SQSTM1, Related to Figures 5 and 6, Respectively (A) (Top panel) Complex formation between HA-RNF26 and either GFP-TAX1BP1 or its mutants UBZ∗ and UBZ2∗. (bottom panel) Complex formation between RFP-RNF26 and either FLAG-EPS15 or its mutant UIM∗. WCL: whole-cell lysate. For quantification of complex formation under the above and other conditions, see Figure 5D. (B) HeLa cells expressing active (top panel) or inactive (bottom panel) HA-RNF26 (red) along GFP-USP15 or its inactive mutant GFP-C269S (green) were stained for endogenous SQSTM1 (blue). A three-color overlay of a representative cell is shown in the top image for each condition and a zoom-in of the indicated perinuclear (PN) region for SQSTM1 and HA-RNF26 or its mutants is indicated below. Arrows point toward locations of colocalization of RNF26 and SQSTM1. Quantification appears in Figure 6D; n = 2, scale bar, 10 μm.
Figure S6
Vesicle Adaptors EPS15, TOLLIP, and TAX1BP1 Colocalize with SQSTM1 at RNF26-Positive Sites on the ER, Related to Figure 7 (A) Co-localization of GFP-TOLLIP (top panels), GFP-EPS15 (middle panels) or GFP-TAX1BP1 (bottom panels) with wild-type HA-RNF26 (red) versus catalytically inactive I382R (red) and SQSTM1 (blue) is shown as representative 3-color overlays and insets with their corresponding individual channels. Co-staining against LC3 is shown to the right. For dynamics of adaptor-selected vesicles associated with the RNF26/SQSTM1 complex see Figures 7 and S7. (B) Quantification of (A). Co-localization (Mander’s overlap) of either RNF26 (HA, black bars) or SQSTM1 (white bars) with GFP-TOLLIP (left), GFP-EPS15 (middle) or GFP-TAX1BP1 (right) is given for cells expressing the active (WT) or inactive (IR) form of RNF26. n = 2. (C) Complex formation between endogenous SQSTM1 and either FLAG-TOLLIP or its mutant CUE∗. WCL: Whole-cell lysate. Scale bar, 10 μm; n = # independent experiments; shown are the mean + error bars = SD.
Figure S7
RNF26/SQSTM1 Complex Retains Adaptor-Selected Vesicles in the Perinuclear Cloud, Related to Figure 7 (A) Overlay confocal frame insets of selected perinuclear (PN) and peripheral (PP) regions from time lapses of HeLa cells co-expressing TRQ-SQSTM1 (blue) and GFP-TAX1BP1 (top, green), GFP-TOLLIP (middle and bottom, green) in the presence of either RFP-RNF26 (red) or RFP-ΔRING (red), as indicated. Quantification appears in Figure 7C. See also Movies S6A and S6B. (B) Schematic illustration of the suggested interaction between RNF26 and RNF26-ΔRING with SQSTM1 and the adaptors on vesicles. Colors correspond to the proteins as shown in (A). Snap shots of HeLa cells expressing RFP-RNF26 (top panels, red) or mutant RFP-RNF26ΔRING (bottom panel, red), TRQ-ubiquitin (blue) and GFP-TOLLIP (green). Three-color zoom-ins and Ubiquitin single (gray) images of a time lapse of the perinuclear region are shown. Quantification appears in Figure 7C. Scale bar, 10μm.
Comment in
- Cloud storage for endosomes.
Dellibovi-Ragheb T, Altan-Bonnet N. Dellibovi-Ragheb T, et al. EMBO J. 2016 Aug 15;35(16):1724-5. doi: 10.15252/embj.201695080. Epub 2016 Jul 4. EMBO J. 2016. PMID: 27378788 Free PMC article.
Similar articles
- The ER-embedded UBE2J1/RNF26 ubiquitylation complex exerts spatiotemporal control over the endolysosomal pathway.
Cremer T, Jongsma MLM, Trulsson F, Vertegaal ACO, Neefjes J, Berlin I. Cremer T, et al. Cell Rep. 2021 Jan 19;34(3):108659. doi: 10.1016/j.celrep.2020.108659. Cell Rep. 2021. PMID: 33472082 - Stop or Go? Endosome Positioning in the Establishment of Compartment Architecture, Dynamics, and Function.
Neefjes J, Jongsma MML, Berlin I. Neefjes J, et al. Trends Cell Biol. 2017 Aug;27(8):580-594. doi: 10.1016/j.tcb.2017.03.002. Epub 2017 Mar 28. Trends Cell Biol. 2017. PMID: 28363667 Review. - ER contact sites direct late endosome transport.
Wijdeven RH, Jongsma ML, Neefjes J, Berlin I. Wijdeven RH, et al. Bioessays. 2015 Dec;37(12):1298-302. doi: 10.1002/bies.201500095. Epub 2015 Oct 6. Bioessays. 2015. PMID: 26440125 Review. - RNF26 binds perinuclear vimentin filaments to integrate ER and endolysosomal responses to proteotoxic stress.
Cremer T, Voortman LM, Bos E, Jongsma ML, Ter Haar LR, Akkermans JJ, Talavera Ormeño CM, Wijdeven RH, de Vries J, Kim RQ, Janssen GM, van Veelen PA, Koning RI, Neefjes J, Berlin I. Cremer T, et al. EMBO J. 2023 Sep 18;42(18):e111252. doi: 10.15252/embj.2022111252. Epub 2023 Jul 31. EMBO J. 2023. PMID: 37519262 Free PMC article. - Retrograde transport: two (or more) roads diverged in an endosomal tree?
Johannes L, Wunder C. Johannes L, et al. Traffic. 2011 Aug;12(8):956-62. doi: 10.1111/j.1600-0854.2011.01200.x. Epub 2011 Apr 28. Traffic. 2011. PMID: 21463456 Review.
Cited by
- USP15 Deubiquitinates TUT1 Associated with RNA Metabolism and Maintains Cerebellar Homeostasis.
Kim J, Nakamura J, Hamada C, Taketomi T, Yano S, Okajima T, Kashiwabara SI, Baba T, Sato B, Chiba T, Tsuruta F. Kim J, et al. Mol Cell Biol. 2020 Oct 13;40(21):e00098-20. doi: 10.1128/MCB.00098-20. Print 2020 Oct 13. Mol Cell Biol. 2020. PMID: 32839293 Free PMC article. - The Autophagy Receptor TAX1BP1 (T6BP) improves antigen presentation by MHC-II molecules.
Sarango G, Richetta C, Pereira M, Kumari A, Ghosh M, Bertrand L, Pionneau C, Le Gall M, Grégoire S, Jeger-Madiot R, Rosoy E, Subra F, Delelis O, Faure M, Esclatine A, Graff-Dubois S, Stevanović S, Manoury B, Ramirez BC, Moris A. Sarango G, et al. EMBO Rep. 2022 Dec 6;23(12):e55470. doi: 10.15252/embr.202255470. Epub 2022 Oct 10. EMBO Rep. 2022. PMID: 36215666 Free PMC article. - Lysosomal positioning diseases: beyond substrate storage.
Scerra G, De Pasquale V, Scarcella M, Caporaso MG, Pavone LM, D'Agostino M. Scerra G, et al. Open Biol. 2022 Oct;12(10):220155. doi: 10.1098/rsob.220155. Epub 2022 Oct 26. Open Biol. 2022. PMID: 36285443 Free PMC article. Review. - Cloud storage for endosomes.
Dellibovi-Ragheb T, Altan-Bonnet N. Dellibovi-Ragheb T, et al. EMBO J. 2016 Aug 15;35(16):1724-5. doi: 10.15252/embj.201695080. Epub 2016 Jul 4. EMBO J. 2016. PMID: 27378788 Free PMC article. - UBTD1 regulates ceramide balance and endolysosomal positioning to coordinate EGFR signaling.
Torrino S, Tiroille V, Dolfi B, Dufies M, Hinault C, Bonesso L, Dagnino S, Uhler J, Irondelle M, Gay AS, Fleuriot L, Debayle D, Lacas-Gervais S, Cormont M, Bertero T, Bost F, Gilleron J, Clavel S. Torrino S, et al. Elife. 2021 Apr 22;10:e68348. doi: 10.7554/eLife.68348. Elife. 2021. PMID: 33884955 Free PMC article.
References
- Ankem G., Mitra S., Sun F., Moreno A.C., Chutvirasakul B., Azurmendi H.F., Li L., Capelluto D.G. The C2 domain of Tollip, a Toll-like receptor signalling regulator, exhibits broad preference for phosphoinositides. Biochem. J. 2011;435:597–608. - PubMed
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous