Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease - PubMed (original) (raw)
Comparative Study
Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease
Brian Lipworth et al. Heart. 2016.
Abstract
Objective: Although β-blockers are an established therapy in heart failure (HF) guidelines, including for patients with chronic obstructive pulmonary disease (COPD), there remain concerns regarding bronchoconstriction even with cardioselective β-blockers. We wished to assess the real-life use of β-blockers for patients with HF and comorbid COPD.
Methods: We evaluated data from the Optimum Patient Care Research Database over a period of 1 year for co-prescribing of β-blockers with either an ACE inhibitor (ACEI) or angiotensin-2 receptor blocker (ARB) in patients with HF alone versus HF+COPD. Association with inhaler therapy was also evaluated.
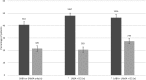
Results: We identified 89 861 patients with COPD, 24 237 with HF and 10 853 with both conditions. In patients with HF+COPD, the mean age was 79 years; 60% were male, and 27% had prior myocardial infarction. Of patients with HF+COPD, 22% were taking a β-blocker in conjunction with either ACEI/ARB (n=2416) compared with 41% of patients with HF only (n=10 002) (adjusted OR 0.54, 95% CI 0.51 to 0.58, p<0.001). Among HF+COPD patients taking inhaled corticosteroid (ICS) with long-acting β-agonist (LABA) and long-acting muscarinic antagonist, 27% of patients were taking an ACEI/ARB with β-blockers (n=778) versus 46% taking an ACEI/ARB without β-blockers (n=1316). Corresponding figures for those patients taking ICS/LABA were 20% (n=583) versus 48% (n=1367), respectively.
Conclusions: These data indicate a substantial unmet need for patients with COPD who should be prescribed β-blockers more often for concomitant HF.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
BL reports grants and personal fees from Chiesi, personal fees from Boerhingher Ingelheim, grants and personal fees from Meda, grants and personal fees from Teva, grants from Janssen, grants from AstraZeneca, grants from Roche, outside the submitted work; VT reports other from Cambridge Research Support, outside the submitted work; JL reports other from Observational and Pragmatic Research Institute, during the conduct of the study; JM reports other from Research in Real Life, outside the submitted work; DBP has board membership with Aerocrine, Almirall, Amgen, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis International AG and Teva. Consultancy: A Almirall, Amgen, AstraZeneca plc, Boehringer Ingelheim, Chiesi, GlaxoSmithKline plc, Meda, Mundipharma, Napp, Novartis International AG, Pfizer and Teva; Grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL, Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline plc, Meda, Merck & Co., Mundipharma, Napp, Novartis International AG, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline plc, Kyorin, Meda, Merck & Co., Mundipharma, Novartis International AG, Pfizer, SkyePharma, Takeda and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL; Payment for the development of educational materials: GlaxoSmithKline plc, Novartis International AG; Stock/Stock options: Shares in AKL which produces phytopharmaceuticals and owns 80% of Research in Real Life and its subsidiary social enterprise Optimum Patient Care; received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis International AG and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva and Zentiva; Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014).
Figures
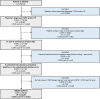
Figure 1
Patient inclusion and exclusion criteria. COPD, chronic obstructive pulmonary disorder; HF, heart failure; OPCRD, Optimum Patient Care Research Database.
Figure 2
Prescription of β-blocker (BB) and ACEI (ACE inhibitor) or angiotensin-2 receptor blocker (ARB) for patients with heart failure (HF) alone versus patients with HF and chronic obstructive pulmonary disease (COPD)—showing data for all patients and split by New York Heart Association (NYHA) class. Data for adjusted ORs are shown with 95% CI.
Figure 3
Use of β-blockers in chronic obstructive pulmonary disease/heart failure according to inhaler therapy. Dark bars depict patients taking ACE inhibitor (ACEI)/ angiotensin-2 receptor blocker (ARB) without β-blockers, while lighter bars depict patients taking ACEI/ARB with β-blockers. The absolute numbers of patients are also shown. Error bars depict 95%CI. Also see table 2 for additional numerical data. ICS, inhaled corticosteroids; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β-2 agonist; SAMA, short-acting muscarinic antagonist.
Comment in
- It is important to distinguish between HFrEF and HFpEF when interpreting these data.
Lipworth B, Skinner D, Devereux G, Thomas V, Ling Zhi Jie J, Martin J, Carter V, Price DB. Lipworth B, et al. Heart. 2016 Dec 1;102(23):1934. doi: 10.1136/heartjnl-2016-310557. Heart. 2016. PMID: 27836947 No abstract available. - It is important to distinguish between HFrEF and HFpEF when interpreting these data.
Cunnington C. Cunnington C. Heart. 2016 Dec 1;102(23):1934. doi: 10.1136/heartjnl-2016-310579. Heart. 2016. PMID: 27836948 No abstract available.
Similar articles
- Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns.
Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, Rossi A, Hutton C, Ashton VL, Stewart R, Bichel K. Price D, et al. Int J Chron Obstruct Pulmon Dis. 2014 Aug 27;9:889-904. doi: 10.2147/COPD.S62750. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 25210450 Free PMC article. - Prevalence and management of COPD and heart failure comorbidity in the general practitioner setting.
Pirina P, Martinetti M, Spada C, Zinellu E, Pes R, Chessa E, Fois AG, Miravitlles M; COPD-HF Study Group. Pirina P, et al. Respir Med. 2017 Oct;131:1-5. doi: 10.1016/j.rmed.2017.07.059. Epub 2017 Jul 25. Respir Med. 2017. PMID: 28947013 - Diagnostic and Therapeutic Gaps in Patients With Heart Failure and Chronic Obstructive Pulmonary Disease.
Canepa M, Franssen FME, Olschewski H, Lainscak M, Böhm M, Tavazzi L, Rosenkranz S. Canepa M, et al. JACC Heart Fail. 2019 Oct;7(10):823-833. doi: 10.1016/j.jchf.2019.05.009. Epub 2019 Sep 11. JACC Heart Fail. 2019. PMID: 31521680 Review. - Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids.
Asche CV, Leader S, Plauschinat C, Raparla S, Yan M, Ye X, Young D. Asche CV, et al. Int J Chron Obstruct Pulmon Dis. 2012;7:201-9. doi: 10.2147/COPD.S25805. Epub 2012 Mar 15. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 22500120 Free PMC article. - Role of Long-Acting Muscarinic Antagonist/Long-Acting β2-Agonist Therapy in Chronic Obstructive Pulmonary Disease.
Petite SE. Petite SE. Ann Pharmacother. 2017 Aug;51(8):696-705. doi: 10.1177/1060028017705149. Epub 2017 Apr 14. Ann Pharmacother. 2017. PMID: 28410560 Review.
Cited by
- Adequacy of Therapy for People with Both COPD and Heart Failure in the UK: Historical Cohort Study.
Kostikas K, Rhee CK, Hurst JR, Agostoni P, Cao H, Fogel R, Jones R, Kocks JWH, Mezzi K, Wan Yau Ming S, Ryan R, Price DB. Kostikas K, et al. Pragmat Obs Res. 2020 Jun 2;11:55-66. doi: 10.2147/POR.S250451. eCollection 2020. Pragmat Obs Res. 2020. PMID: 32581622 Free PMC article. - Therapeutic Approaches for Chronic Obstructive Pulmonary Disease (COPD) Exacerbations.
Rosenwasser Y, Berger I, Loewy ZG. Rosenwasser Y, et al. Pathogens. 2022 Dec 10;11(12):1513. doi: 10.3390/pathogens11121513. Pathogens. 2022. PMID: 36558847 Free PMC article. Review. - Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-Current Considerations for Clinical Practice.
Trinkmann F, Saur J, Borggrefe M, Akin I. Trinkmann F, et al. J Clin Med. 2019 Jan 10;8(1):69. doi: 10.3390/jcm8010069. J Clin Med. 2019. PMID: 30634565 Free PMC article. Review. - Role of Comorbidities in Treatment and Outcomes after Chronic Obstructive Pulmonary Disease Exacerbations.
Spece LJ, Epler EM, Donovan LM, Griffith MF, Collins MP, Feemster LC, Au DH. Spece LJ, et al. Ann Am Thorac Soc. 2018 Sep;15(9):1033-1038. doi: 10.1513/AnnalsATS.201804-255OC. Ann Am Thorac Soc. 2018. PMID: 30079748 Free PMC article. - Real-world effectiveness of medications on survival in patients with COPD-heart failure overlap.
Su VY, Yang YH, Perng DW, Tsai YH, Chou KT, Su KC, Su WJ, Chen PC, Yang KY. Su VY, et al. Aging (Albany NY). 2019 Jun 7;11(11):3650-3667. doi: 10.18632/aging.102004. Aging (Albany NY). 2019. PMID: 31175265 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous