The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity - PubMed (original) (raw)
The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity
Iordan Iordanov et al. Elife. 2016.
Abstract
Transient Receptor Potential Melastatin 2 (TRPM2) is a Ca(2+)-permeable cation channel essential for immunocyte activation, insulin secretion, and postischemic cell death. TRPM2 is activated by ADP ribose (ADPR) binding to its C-terminal cytosolic NUDT9-homology (NUDT9H) domain, homologous to the soluble mitochondrial ADPR pyrophosphatase (ADPRase) NUDT9. Reported ADPR hydrolysis classified TRPM2 as a channel-enzyme, but insolubility of isolated NUDT9H hampered further investigations. Here we developed a soluble NUDT9H model using chimeric proteins built from complementary polypeptide fragments of NUDT9H and NUDT9. When expressed in E.coli, chimeras containing up to ~90% NUDT9H sequence remained soluble and were affinity-purified. In ADPRase assays the conserved Nudix-box sequence of NUDT9 proved essential for activity (kcat~4-9s(-1)), that of NUDT9H did not support catalysis. Replacing NUDT9H in full-length TRPM2 with soluble chimeras retained ADPR-dependent channel gating (K1/2~1-5 μM), confirming functionality of chimeric domains. Thus, TRPM2 is not a 'chanzyme'. Chimeras provide convenient soluble NUDT9H models for structural/biochemical studies.
Keywords: ADPR; E. coli; NUDT9H domain; TRPM2; biochemistry; biophysics; chanzyme; chimera; structural biology; xenopus.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
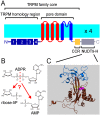
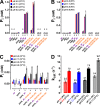
Figure 1.. The ligand-binding NUDT9H domain in the context of TRPM2.
(A) Topology map of a TRPM2 monomer, showing the N-terminal TRPM homology region (dark blue), the six transmembrane helices which form the pore domain (red and blue), and a C-terminal short coiled-coil region (CCR, yellow) followed by the NUDT9H domain (gray) which is homologous to the soluble mitochondrial ADPRase NUDT9. (B) Hydrolysis of ADPR to AMP and R5P: reaction catalyzed by mitochondrial NUDT9 and reportedly also by the TRPM2 NUDT9H domain. (C) Crystal structure of mitochondrial NUDT9 (PDB ID: 1QVJ) illustrating Cap (blue) and Core (brown) subdomains of the protein, with the hydrolysis product R5P (yellow) and two Mg2+ ions (magenta) bound. DOI:
http://dx.doi.org/10.7554/eLife.17600.003
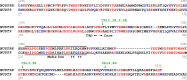
Figure 2.. Alignment of NUDT9H and NUDT9 sequences.
Aligned (Corpet, 1988) sequences of NUDT9H and NUDT9, highlighting identical (red) and similar (blue) residues. The approximate border between Cap and Core subdomains of the proteins is indicated by two black arrows. Black line identifies the Nudix box, asterisks denote residues critical for the catalytic activity of NUDT9. Green arrows and labels indicate transition points for sequence swapping in each chimera (see also Figure 3). DOI:
http://dx.doi.org/10.7554/eLife.17600.004
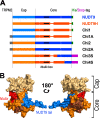
Figure 3.. Representation of the various recombinant proteins used in this study.
(A) Aligned block diagrams of recombinant constructs with segment lengths realistically scaled to reflect their actual sizes (numbers of residues). The Cap and Core segments of NUDT9 (blue) and NUDT9H (orange) are separated by a vertical black line. Gaps emerging from the alignment of the two sequences (Figure 2) are presented as thick lines with the respective color. The 15 amino-acid stretch from the upstream cytosolic linker sequence of TRPM2 (gray), present in some chimeras, contains a native _BlpI_-site which allows subcloning the domain into full-length TRPM2. Some constructs contain a C-terminal hexahistidine tag (green) or Twin-Strep tag (magenta). Nudix boxes within Core segments are indicated as black lines (see also Table 1). (B) NUDT9 fragments replaced in the chimeras by NUDT9H sequence, mapped onto surface representation of NUDT9 crystal structure (PDB ID: 1Q33). Coloring highlights progressive replacement of NUDT9 sequence with NUDT9H sequence in chimeric constructs. The Cap subdomain (light brown) is replaced in Chi2 and Chi2A. A fragment from the Core (orange), containing the Nudix box (red), is also replaced in Chi3S. Additional Core fragment (dark brown) further extends the NUDT9H sequence in Chi4S, leaving only 36 C-terminal amino acids from NUDT9 (blue). DOI:
http://dx.doi.org/10.7554/eLife.17600.005
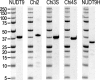
Figure 3—figure supplement 1.. Purified recombinant proteins.
Coomassie-stained SDS PAGE gels of the five purified recombinant proteins (mitochondrial NUDT9, three soluble chimeric constructs, and the NUDT9H domain of TRPM2), as indicated above each gel slice. Molecular weights for marker ladder bands (Precision, Bio-Rad) are labeled in kDa. DOI:
http://dx.doi.org/10.7554/eLife.17600.006
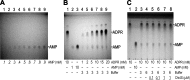
Figure 4.. Enzymatic activity of NUDT9, NUDT9H, and chimeras.
(A–E) 10 mM ADPR (lane C1) and 10 mM AMP (lane C2) controls in water. Lanes 1–6 show reaction mixtures of 1 μM purified NUDT9 (A), Chi2 (B), Chi3S (C), Chi4S (D), or NUDT9H (E), incubated with 10 mM ADPR and 16 mM MgCl2 in 50 mM RB1-6, respectively, for 2 hr at room temperature. (F) Top slice: TLC-detection limit for AMP: 0.1–0.9 mM water-dissolved AMP in 0.1-mM incrementing steps. Middle slice: ADPR self-degradation. The slice shows only the AMP position of (from left to right): 10 mM ADPR control (in water), 1 mM AMP control (in water), 10 mM AMP control (in water), and 1, 2, 5, 10, 15 and 20 mM ADPR incubated for 24 hr at room temperature in RB3 (pH 8.5) (last six lanes). Bottom slice: ADPR self-degradation in the absence and presence of Chi4S, showing only the AMP position. From left to right: 10 mM ADPR control (in water); 10 mM AMP control (in water); 10 mM ADPR in RB3 (pH 8.5); 10 mM ADPR in RB6 (pH 8.5/NaCl); 0.1 μM Chi4S + 10 mM ADPR in RB3; 0.1 μM Chi4S + 10 mM ADPR in RB6; 1 μM Chi4S + 10 mM ADPR in RB3; 1 μM Chi4S + 10 mM ADPR in RB6. Except for the controls in lanes 1–2, all samples also contained 16 mM MgCl2, and were incubated for 24 hr at room temperature. In all Panels 1-μl aliquots were loaded for each sample. The three slices of Panel F can be seen as full-scale TLC plates in Figure 4—figure supplement 1. DOI:
http://dx.doi.org/10.7554/eLife.17600.008
Figure 4—figure supplement 1.. Full-scale TLC images of the slices shown in Panel F on Figure 4.
(A) Image of entire TLC plate from which the top slice in Figure 4F was excised: TLC-detection limit for AMP. Lanes 1–9 show 0.1–0.9 mM water-dissolved AMP, respectively, in 0.1-mM incrementing steps. (B) Image of entire TLC plate from which the middle slice in Figure 4F was excised: ADPR self-degradation. Lane 1: 10 mM ADPR control (in water); lane 2: 1 mM AMP control (in water); lane 3: 10 mM AMP control (in water); lanes 4–9: 1, 2, 5, 10, 15 and 20 mM ADPR, respectively, incubated for 24 hr at room temperature in RB3 (pH 8.5). (C) Image of entire TLC plate from which the bottom slice in Figure 4F was excised. ADPR self-degradation in the absence and presence of Chi4S. Lane 1: 10 mM ADPR control (in water); lane 2: 10 mM AMP control (in water); lane 3: 10 mM ADPR in RB3 (pH 8.5); lane 4: 10 mM ADPR in RB6 (pH 8.5/NaCl); lane 5: 0.1 µM Chi4S + 10 mM ADPR in RB3; lane 6: 0.1 µM Chi4S + 10 mM ADPR in RB6; lane 7: 1 µM Chi4S + 10 mM ADPR in RB3; lane 8: 1 µM Chi4S + 10 mM ADPR in RB6. Except for the controls in lanes 1–2, all samples also contained 16 mM MgCl2, and were incubated for 24 hr at room temperature. In all Panels 1-μl aliquots were loaded for each sample. DOI:
http://dx.doi.org/10.7554/eLife.17600.009
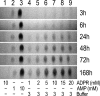
Figure 4—figure supplement 2.. Spontaneous ADPR degradation at basic pH, monitored with TLC.
The six TLC slices show only the AMP position of 10 mM ADPR control in water (lane 1), 1 mM AMP control in water (lane 2), 10 mM AMP control in water (lane 3), and 1, 2, 5, 10, 15 and 20 mM ADPR, respectively, in RB3 (pH 8.5) (lanes 4–9). The ADPR samples were incubated at room temperature for different time durations, as indicated to the right of each slice. In all slices 1-μl aliquots were loaded for each sample. The 24h-slice is the same experiment shown in Figure 4F, middle slice (and in Figure 4—figure supplement 1B). DOI:
http://dx.doi.org/10.7554/eLife.17600.010
Figure 5.. Enzyme kinetics of Chi2 and NUDT9.
(A) Representative TLC images showing the time courses (in minutes) of hydrolysis of ADPR (10 mM) by 0.5 μM Chi2 (top) or NUDT9 (bottom) at 3 different pH values (RB1, RB2 and RB3). The slices show only the position of the AMP spots. Control 1 (C1): 5 mM AMP in water. Control 2 (C2): 10 mM AMP in water. (B) AMP accumulation during ADPR hydrolysis by Chi2 (circles) and NUDT9 (triangles) at 3 different pH values. Densities of AMP spots (such as those shown in Panel A) were normalized to the density of the control AMP spot (5 mM) on the same TLC sheet. For each time point mean ± SEM from 3 experiments is shown. Straight lines are linear regression fits to the averaged data points. (C) Estimates of _k_cat (s−1) for both proteins at each pH, calculated from the fitted slopes in B (mean ± SEM; mean values are also printed above each bar). DOI:
http://dx.doi.org/10.7554/eLife.17600.011
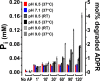
Figure 6.. Enzymatic activity of NUDT9, Chi4S, and NUDT9H measured with the Pi assay.
(A–B) Pi (in mM) liberated in sample mixtures containing, as indicated, AP (5–6 U alkaline phosphatase), ADPR (1 mM), AMP (1 mM), R5P (1 mM), and either 5 or 50 nM protein (NUDT9, Chi4S, or NUDT9H), and incubated for 30 min at pH 6.5, 7.1, 8.5, or 9.0 (color coded) at either 37°C (A) or room temperature (RT) (B). Asterisks in A denote samples replotted with an expanded ordinate in Panel C. Bars represent mean ± SEM from 3 independent experiments. (C) Expanded display of samples denoted with asterisks in Panel A. (D) Estimated _k_cat values (in s−1) for NUDT9 under each experimental condition (pH and temperature), calculated (see Materials and methods) from [Pi] liberated in the samples containing 5 nM NUDT9 (group 8) in Panels A and B. DOI:
http://dx.doi.org/10.7554/eLife.17600.012
Figure 6—figure supplement 1.. Spontaneous ADPR degradation at basic pH, monitored with Pi assay.
Pi assay (see Materials and methods) for 2 mM ADPR in the absence of an ADPRase, incubated at various pH and temperatures (as labeled) for the indicated time (in minutes). 'No AP' – Alkaline phosphatase was not added, and incubation time was 120 min. The two ordinates represent the concentration of liberated Pi (in mM) (left), and the corresponding mol% of degraded ADPR (right). Bars represent mean ± SEM from 3 independent experiments. DOI:
http://dx.doi.org/10.7554/eLife.17600.013
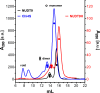
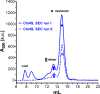
Figure 6—figure supplement 2.. Size exclusion chromatograms of NUDT9, HUDT9H, and Chi4S.
Size exclusion chromatography elution profiles, detected as absorbance at 280 nm, of affinity-purified NUDT9 (black), Chi4S (blue), and NUDT9H (red; note 10-fold expanded ordinate on the right). Peaks corresponding to monomers and dimers are labeled DOI:
http://dx.doi.org/10.7554/eLife.17600.014
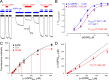
Figure 7.. ADPR-induced channel activity of full-length TRPM2 chimeras.
(A–D) Macroscopic currents in inside-out patches excised from Xenopus laevis oocytes expressing (A) T5L-TRPM2, (B) T5L-TRPM2-Chi2, (C) T5L-TRPM2-Chi3, and (D) T5L-TRPM2-Chi4, elicited by superfusion with increasing concentrations of ADPR in the presence of saturating Ca2+ (black bars). Membrane potential was −20 mV; current relaxation time courses following removal of either Ca2+ or ADPR were fitted by single exponentials (colored lines) with time constants indicated. (E) Fractional current activation of the constructs in A–D by 1 μM ADPR; mean steady currents in 1 μM ADPR were divided by those in 32 μM ADPR in the same patch (mean ± SEM, n≥8). (F–G) Time constants of current relaxation upon sudden removal of ADPR (F) or Ca2+ (G) for the constructs in A–D, obtained from single-exponential fits (mean ± SEM, n≥5). (H) Dose response curve of fractional current activation for T5L-TRPM2 (gray) and T5L-TRPM2-Chi2 (light blue); mean steady currents in various test [ADPR] were divided by those in 32 μM ADPR in the same patch. Symbols represent mean ± SEM (n≥5), solid lines are fits to the Hill equation with midpoints printed in the panel. DOI:
http://dx.doi.org/10.7554/eLife.17600.015
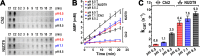
Figure 8.. Full-length TRPM2 and the isolated Chi4S protein bind ε-ADPR with similar affinities.
(A) Macroscopic inward T5L-TRPM2 current in inside-out patch excised from Xenopus laevis oocyte, elicited at -20 mV by repeated exposures to saturating Ca2+ (black bars) and 1 or 100 μM ADPR (blue bars), in the absence or presence of 100 μM ε-ADPR (red bars). (B) Dose-dependence of TRPM2 activation by ADPR in the absence (blue symbols and fit line) and presence (violet symbols and fit line) of 100 μM ε-ADPR (mean ± SEM, n≥7). Printed parameters _K_ADPR, n, and _K_ε-ADPR reflect the apparent affinity and Hill slope for ADPR binding, and _K_I for ε-ADPR binding, respectively, obtained from the fits (see Materials and methods). (C) Fluorescence intensity (arbitrary units, Ex: 310 nm, Em: 420 nm) of ε-ADPR at various total concentrations in the absence of protein (black symbols), or in the presence of 100 μM of either BSA (gray symbols) or Chi4S (red symbols). Symbols plot mean ± SD from four, two, and two independent titrations, respectively, for buffer (black), BSA (gray), and Chi4S (red). Black line is a smooth fit of the black symbols by a three-parameter empirical mathematical function, and was used as a calibration curve to determine free [ε-ADPR] in the presence of Chi4S, assuming negligible fluorescence of the bound analog (red projection lines). (D) Plot of upper estimate of free [ε-ADPR] as a function of total [ε-ADPR] in the presence of 100 μM Chi4S (red symbols), fitted (red line) to a simple binding equation (see Materials and methods) to obtain an upper estimate of _K_d (see panel). Black line illustrates free [ε-ADPR] expected in the absence of binding. DOI:
http://dx.doi.org/10.7554/eLife.17600.016
Figure 8—figure supplement 1.. Lack of effect of five-fold concentration on Chi4S solubility.
Size exclusion chromatography elution profiles, detected as absorbance at 280 nm. Chi4S was expressed and purified as described in Materials and methods, using Steptactin affinity chromatography followed by size exclusion chromatography (solid blue profile). The pooled monomeric fraction was left overnight at 4°C, and then concentrated five-fold, to ~100 μM, using a 10 kDa molecular weight cutoff Vivaspin column (Sartorius). This concentrated protein was then subjected to a second round of size exclusion chromatography (dashed blue profile). DOI:
http://dx.doi.org/10.7554/eLife.17600.017
Similar articles
- Ligand-induced activation of human TRPM2 requires the terminal ribose of ADPR and involves Arg1433 and Tyr1349.
Fliegert R, Watt JM, Schöbel A, Rozewitz MD, Moreau C, Kirchberger T, Thomas MP, Sick W, Araujo AC, Harneit A, Potter BVL, Guse AH. Fliegert R, et al. Biochem J. 2017 Jun 16;474(13):2159-2175. doi: 10.1042/BCJ20170091. Biochem J. 2017. PMID: 28515263 Free PMC article. - Functional importance of NUDT9H domain and N-terminal ADPR-binding pocket in two species variants of vertebrate TRPM2 channels.
Kühn FJP, Ehrlich W, Barth D, Kühn C, Lückhoff A. Kühn FJP, et al. Sci Rep. 2019 Dec 16;9(1):19224. doi: 10.1038/s41598-019-55232-5. Sci Rep. 2019. PMID: 31844070 Free PMC article. - ADP-Ribose Activates the TRPM2 Channel from the Sea Anemone Nematostella vectensis Independently of the NUDT9H Domain.
Kühn FJ, Kühn C, Winking M, Hoffmann DC, Lückhoff A. Kühn FJ, et al. PLoS One. 2016 Jun 22;11(6):e0158060. doi: 10.1371/journal.pone.0158060. eCollection 2016. PLoS One. 2016. PMID: 27333281 Free PMC article. - Different Principles of ADP-Ribose-Mediated Activation and Opposite Roles of the NUDT9 Homology Domain in the TRPM2 Orthologs of Man and Sea Anemone.
Kühn F, Kühn C, Lückhoff A. Kühn F, et al. Front Physiol. 2017 Oct 31;8:879. doi: 10.3389/fphys.2017.00879. eCollection 2017. Front Physiol. 2017. PMID: 29163217 Free PMC article. Review. - TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose.
Kühn FJ, Heiner I, Lückhoff A. Kühn FJ, et al. Pflugers Arch. 2005 Oct;451(1):212-9. doi: 10.1007/s00424-005-1446-y. Epub 2005 Jun 11. Pflugers Arch. 2005. PMID: 15952035 Review.
Cited by
- A structural overview of the ion channels of the TRPM family.
Huang Y, Fliegert R, Guse AH, Lü W, Du J. Huang Y, et al. Cell Calcium. 2020 Jan;85:102111. doi: 10.1016/j.ceca.2019.102111. Epub 2019 Nov 24. Cell Calcium. 2020. PMID: 31812825 Free PMC article. Review. - TRPM2: bridging calcium and ROS signaling pathways-implications for human diseases.
Maliougina M, El Hiani Y. Maliougina M, et al. Front Physiol. 2023 Jul 27;14:1217828. doi: 10.3389/fphys.2023.1217828. eCollection 2023. Front Physiol. 2023. PMID: 37576339 Free PMC article. Review. - TRPM2 ion channels steer neutrophils towards a source of hydrogen peroxide.
Morad H, Luqman S, Tan CH, Swann V, McNaughton PA. Morad H, et al. Sci Rep. 2021 Apr 29;11(1):9339. doi: 10.1038/s41598-021-88224-5. Sci Rep. 2021. PMID: 33927223 Free PMC article. - Characterization and Optimization of the Novel Transient Receptor Potential Melastatin 2 Antagonist tatM2NX.
Cruz-Torres I, Backos DS, Herson PS. Cruz-Torres I, et al. Mol Pharmacol. 2020 Feb;97(2):102-111. doi: 10.1124/mol.119.117549. Epub 2019 Nov 26. Mol Pharmacol. 2020. PMID: 31772034 Free PMC article. - TRPM2: a candidate therapeutic target for treating neurological diseases.
Belrose JC, Jackson MF. Belrose JC, et al. Acta Pharmacol Sin. 2018 May;39(5):722-732. doi: 10.1038/aps.2018.31. Epub 2018 Apr 19. Acta Pharmacol Sin. 2018. PMID: 29671419 Free PMC article. Review.
References
- Boto AN, Xu W, Jakoncic J, Pannuri A, Romeo T, Bessman MJ, Gabelli SB, Amzel LM. Structural studies of the Nudix GDP-mannose hydrolase from E. coli reveals a new motif for mannose recognition. Proteins: Structure, Function, and Bioinformatics. 2011;79:2455–2466. doi: 10.1002/prot.23069. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous