How antibodies alter the cell entry pathway of dengue virus particles in macrophages - PubMed (original) (raw)
How antibodies alter the cell entry pathway of dengue virus particles in macrophages
Nilda V Ayala-Nunez et al. Sci Rep. 2016.
Abstract
Antibody-dependent enhancement of dengue virus (DENV) infection plays an important role in the exacerbation of DENV-induced disease. To understand how antibodies influence the fate of DENV particles, we explored the cell entry pathway of DENV in the absence and presence of antibodies in macrophage-like P388D1 cells. Recent studies unraveled that both mature and immature DENV particles contribute to ADE, hence, both particles were studied. We observed that antibody-opsonized DENV enters P388D1 cells through a different pathway than non-opsonized DENV. Antibody-mediated DENV entry was dependent on FcγRs, pH, Eps15, dynamin, actin, PI3K, Rab5, and Rab7. In the absence of antibodies, DENV cell entry was FcγR, PI3K, and Rab5-independent. Live-cell imaging of fluorescently-labeled particles revealed that actin-mediated membrane protrusions facilitate virus uptake. In fact, actin protrusions were found to actively search and capture antibody-bound virus particles distantly located from the cell body, a phenomenon that is not observed in the absence of antibodies. Overall, similar results were seen for antibody-opsonized standard and antibody-bound immature DENV preparations, indicating that the maturation status of the virus does not control the entry pathway. Collectively, our findings suggest that antibodies alter the cell entry pathway of DENV and trigger a novel mechanism of initial virus-cell contact.
Figures
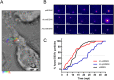
Figure 1. Kinetics of antibody-opsonized DENV entry in macrophages.
Single-particle tracking of DiD-labeled Ab-stdDENV, Ab-prMDENV and stdDENV was performed in P388D1 cells at 1 frame per second. (A) Cell image obtained with DIC optics showing a trajectory of a single DiD-labeled Ab-stdDENV particle. The white arterisk represents the membrane fusion site. Scale bar: 2 μm. (B) Snapshots of a DiD-labeled stdDENV, Ab-stdDENV, and Ab-prMDENV particle at different time points post-infection. Membrane fusion is observed as a sudden increase in fluorescence intensity. The image was artificially modified to show the differences in fluorescence intensity, from low (purple) to high (yellow) intensity. (C) The percentage of fused virus particles calculated as a function of time. In total 35 trajectories were analyzed for Ab-stdDENV, 37 for Ab-prMDENV, and 36 for stdDENV. The time point of membrane fusion was defined as the moment when the DiD intensity doubles.
Figure 2. Antibody-opsonized DENV entry inhibition by endocytic inhibitors.
DiD-labeled DENV (opsonized and non-opsonized) was added in situ to P388D1 cells in the presence or absence of the indicated inhibitors. After 30 min of infection at 37 °C, the cells were washed and snapshots were taken with an oil-immersion 100× objective. (A–D) Representative images upon DiD-DENV infection with and without prior treatment of the cells with NH4Cl (50 mM) and FcR blocker are shown. Scale bar: 12.5 μm. Fusion inhibition was calculated by analyzing the total extent of membrane fusion of DiD-labeled virus with ImageJ. (E–G) Representative images of a fusion assay performed in P388D1 cells electroporated with Eps15-GFP (empty vector D3Δ2 (control) and DNM (E95/295)). Scale bar: 7 μm. (H–J) Fusion inhibition of Eps15 and multiple biochemical inhibitors. An end concentration of 50 μM nystatin, 150 μM dynasore, 200 μM iminodyn-22, 15 μM cytochalasin B, 1 μM latrunculin, 2 μM wortmannin, or 30 μM AS-604850 was used. The percentage of fusion inhibition was calculated with respect to the empty vector or non-treated control, respectively. The average of at least three independent experiments is shown. Error bars represent SEM.
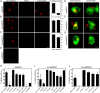
Figure 3. Ab-DENV induction of actin cytoskeleton rearrangement via FcγRs.
DiD-labeled virus (Ab-stdDENV, Ab-prMDENV and stdDENV) was added to eYFP-Actin-expressing P388D1 cells. The cells were kept at 37 °C for live-cell imaging with a spinning disk confocal microscope. Ab-DENV was observed to localize to the (A) cell body, (B) membrane ruffles and (C) actin filopodia during entry. Scale bar: 7 μm. (D) Time series of antibody-opsonized DENV induced cell membrane ruffling. The white arrowhead indicates the place where the ruffle starts. (E–I) Cell movement was quantified over time upon the indicated treatments. (E) Representative images of P388D1 cells under the indicated conditions are shown as the overlays of the cell body outlines of a time-lapse of 20 min. (F–I) The Movement ratio (MR) quantifies the extent of movement of one cell over a frame sequence. See Fig. S7 for more details on how to calculate it. Based on the MR, the movement response was classified as Low (MR < 2), Intermediate (MR = 2–3) or Extensive (MR > 3). For each condition, 15 cells were used for analysis. The gray area indicates the intermediate response. The proportion of cells showing Low, Intermediate, and Extensive response is indicated at the right corner of (E).
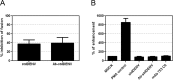
Figure 4. Role of antibody-induced macropinocytosis in virus internalization.
(A) P388D1 cells were treated with EIPA (25 μM) for 1 h at 37 °C. Cells were infected with DiD-labeled stdDENV and Ab-stdDENV and analyzed as in Fig. 2. (B) Fluid phase uptake of Dextran-TxRd upon addition of stdDENV, Ab-stdDENV or mAb # 753 C6. PMA (60 ng/ml) was added as a positive control. All treatments were added 15 min before addition of Dextran-TxRd. Uptake of Dextran (0.4 mg/ml) was allowed for 30 min at 37 °C. The average of five independent experiments is shown. Error bars represent SEM.
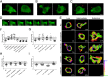
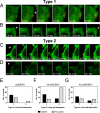
Figure 5. Ab-DENV uptake is mediated by actin protrusions.
The experimental set-up is similar as described in the legend to Fig. 3. (A–D) Representative montages of alternating time-lapse exposures of DiD-DENV and eYFP-Actin during entry. (A,B) Type 1 virus uptake. (C,D) Type 2 virus uptake. DiD-virus is shown in red. eYFP-Actin expressing cells are shown in green. White arrowheads point out single viral particles associated with actin structures. (E–G) Frequency of Type 1 and type 2 uptake in a total of 60 events.
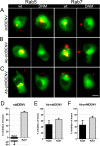
Figure 6. Effect of Rab5 and Rab7 dominant-negative mutants in fusion of Ab-DENV.
DiD-labeled DENV (with and without antibodies) was added in situ to P388D1 cells electroporated with the indicated plasmids. (A–C) Representative examples of membrane fusion. Membrane fusion of DiD-labeled virus is observed as highly fluorescent red puncta in the WT and DNM electroporated cells. All plasmids have GFP as a reporter gene. Scale bar: 7 μm. (D–F) Quantification of the effect of the DNM proteins on viral fusion. For analysis, 85 cells positive for the expression of the plasmids were used. The percentage of inhibition was calculated with respect to the WT plasmid control.
Similar articles
- Antibody-Dependent Dengue Virus Entry Modulates Cell Intrinsic Responses for Enhanced Infection.
Chan CYY, Low JZH, Gan ES, Ong EZ, Zhang SL, Tan HC, Chai X, Ghosh S, Ooi EE, Chan KR. Chan CYY, et al. mSphere. 2019 Sep 18;4(5):e00528-19. doi: 10.1128/mSphere.00528-19. mSphere. 2019. PMID: 31533998 Free PMC article. - Functional genomics screens reveal a role for TBC1D24 and SV2B in antibody-dependent enhancement of dengue virus infection.
Belmont L, Contreras M, Cartwright-Acar CH, Marceau CD, Agrawal A, Levoir LM, Lubow J, Goo L. Belmont L, et al. J Virol. 2024 Nov 19;98(11):e0158224. doi: 10.1128/jvi.01582-24. Epub 2024 Oct 8. J Virol. 2024. PMID: 39377586 Free PMC article. - Blockade of dengue virus entry into myeloid cells by endocytic inhibitors in the presence or absence of antibodies.
Carro AC, Piccini LE, Damonte EB. Carro AC, et al. PLoS Negl Trop Dis. 2018 Aug 9;12(8):e0006685. doi: 10.1371/journal.pntd.0006685. eCollection 2018 Aug. PLoS Negl Trop Dis. 2018. PMID: 30092029 Free PMC article. - The mechanistic role of antibodies to dengue virus in protection and disease pathogenesis.
Gan ES, Ting DH, Chan KR. Gan ES, et al. Expert Rev Anti Infect Ther. 2017 Feb;15(2):111-119. doi: 10.1080/14787210.2017.1254550. Epub 2016 Nov 11. Expert Rev Anti Infect Ther. 2017. PMID: 27796143 Review. - Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development.
Acosta EG, Bartenschlager R. Acosta EG, et al. Expert Rev Vaccines. 2016;15(4):467-82. doi: 10.1586/14760584.2016.1121814. Epub 2015 Dec 15. Expert Rev Vaccines. 2016. PMID: 26577689 Review.
Cited by
- Dissecting strategies to tune the therapeutic potential of SARS-CoV-2-specific monoclonal antibody CR3022.
Atyeo C, Slein MD, Fischinger S, Burke J, Schäfer A, Leist SR, Kuzmina NA, Mire C, Honko A, Johnson R, Storm N, Bernett M, Tong P, Zuo T, Lin J, Zuiani A, Linde C, Suscovich T, Wesemann DR, Griffiths A, Desjarlais JR, Juelg BD, Goudsmit J, Bukreyev A, Baric R, Alter G. Atyeo C, et al. JCI Insight. 2021 Jan 11;6(1):e143129. doi: 10.1172/jci.insight.143129. JCI Insight. 2021. PMID: 33427208 Free PMC article. - Dengue virus infection - a review of pathogenesis, vaccines, diagnosis and therapy.
Kok BH, Lim HT, Lim CP, Lai NS, Leow CY, Leow CH. Kok BH, et al. Virus Res. 2023 Jan 15;324:199018. doi: 10.1016/j.virusres.2022.199018. Epub 2022 Dec 7. Virus Res. 2023. PMID: 36493993 Free PMC article. Review. - The Role of Host Cytoskeleton in Flavivirus Infection.
Zhang Y, Gao W, Li J, Wu W, Jiu Y. Zhang Y, et al. Virol Sin. 2019 Feb;34(1):30-41. doi: 10.1007/s12250-019-00086-4. Epub 2019 Feb 6. Virol Sin. 2019. PMID: 30725318 Free PMC article. Review. - Hypoxia enhances antibody-dependent dengue virus infection.
Gan ES, Cheong WF, Chan KR, Ong EZ, Chai X, Tan HC, Ghosh S, Wenk MR, Ooi EE. Gan ES, et al. EMBO J. 2017 May 15;36(10):1348-1363. doi: 10.15252/embj.201695642. Epub 2017 Mar 20. EMBO J. 2017. PMID: 28320741 Free PMC article. - Intrinsic ADE: The Dark Side of Antibody Dependent Enhancement During Dengue Infection.
Narayan R, Tripathi S. Narayan R, et al. Front Cell Infect Microbiol. 2020 Oct 2;10:580096. doi: 10.3389/fcimb.2020.580096. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33123500 Free PMC article. Review.
References
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf (2009) (Date of access 06-04-2016). - PubMed
- Guzman M. G., Alvarez M. & Halstead S. B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 158, 1445–59 (2013). - PubMed
- Halstead S. B. Dengue virus-mosquito interactions. Annu. Rev. Entomol. 53, 273–91 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous