Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action - PubMed (original) (raw)
Review
Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action
T P Tim Cushnie et al. Cell Mol Life Sci. 2016 Dec.
Abstract
Efforts to reduce the global burden of bacterial disease and contend with escalating bacterial resistance are spurring innovation in antibacterial drug and biocide development and related technologies such as photodynamic therapy and photochemical disinfection. Elucidation of the mechanism of action of these new agents and processes can greatly facilitate their development, but it is a complex endeavour. One strategy that has been popular for many years, and which is garnering increasing interest due to recent technological advances in microscopy and a deeper understanding of the molecular events involved, is the examination of treated bacteria for changes to their morphology and ultrastructure. In this review, we take a critical look at this approach. Variables affecting antibacterial-induced alterations are discussed first. These include characteristics of the test organism (e.g. cell wall structure) and incubation conditions (e.g. growth medium osmolarity). The main body of the review then describes the different alterations that can occur. Micrographs depicting these alterations are presented, together with information on agents that induce the change, and the sequence of molecular events that lead to the change. We close by highlighting those morphological and ultrastructural changes which are consistently induced by agents sharing the same mechanism (e.g. spheroplast formation by peptidoglycan synthesis inhibitors) and explaining how changes that are induced by multiple antibacterial classes (e.g. filamentation by DNA synthesis inhibitors, FtsZ disruptors, and other types of agent) can still yield useful mechanistic information. Lastly, recommendations are made regarding future study design and execution.
Keywords: Antibiotic; Antiseptic; Disinfectant; Mode of action; SEM; TEM.
Figures
Fig. 1
Scanning electron micrographs of a untreated Escherichia coli JP5128 and b cells of the same strain treated with 200 µg/mL chloramphenicol for 3 h (this concentration and time frame were selected because it achieved near-complete inhibition of protein synthesis). Arrow shows the location of a spheroplast. Bar 1 µm. Images from [97] by permission of Antimicrobial Agents and Chemotherapy (ASM)
Fig. 2
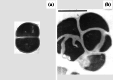
Scanning electron micrographs of a untreated Pseudomonas aeruginosa MB3286 and b cells of the same strain treated with 2 µg/mL (2×MIC) imipenem for 3 h. All of the treated bacteria have changed to ovoid cells. Magnification ×9000. Images from [101] by permission of Innate Immunity (SAGE Publications)
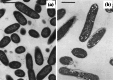
Fig. 3
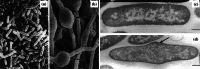
Scanning electron micrographs of a untreated Pseudomonas aeruginosa X48 and b cells of the same strain treated with 32 µg/mL (2×MIC) of the β-lactam BL-P1654 for 4 h, and transmission electron micrographs of c untreated Escherichia coli ATCC 11303 and d cells of the same strain treated with 0.1 µg/mL (1×MIC) mitomycin C for 2 h. The number and length of filamentous cells has increased following treatment. Magnification for a ×2700, b ×2200, c ×18,000, and d ×18,000. Images a and b from [67] by permission of Antimicrobial Agents and Chemotherapy (ASM), and c and d from [133] by permission of the Journal of Bacteriology (ASM)
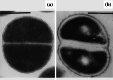
Fig. 4
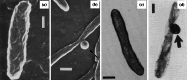
Transmission electron micrographs of a untreated Staphylococcus aureus ATCC 6538P and b cells of the same strain treated with 0.027 µg/mL (0.33×MIC) cephaloridine for 6 h. Treatment has inhibited cell separation, resulting in a pseudomulticellular form. Magnification for a ×23,500 and b ×21,500. Bar 1 µm. Images from [125] by permission of Antimicrobial Agents and Chemotherapy (ASM)
Fig. 5
Scanning electron micrographs of a untreated Pseudomonas aeruginosa MB3286 and b cells of the same strain treated with 2 µg/mL (2×MIC) meropenem for 6 h, and transmission electron micrographs of c untreated Escherichia coli ATCC 12407 and d cells of the same strain treated with 5 µg/mL (~3×MIC) ampicillin for 1 h. Arrows show the location of localized swelling. Magnification for a ×9000, b ×14,000, c ×27,500, and d ×27,000. Bar 0.25 µm. Images a and b from [101] by permission of Innate Immunity (SAGE Publications), and images c and d from [169] by permission of the Journal of Bacteriology (ASM)
Fig. 6
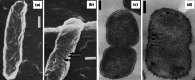
Scanning electron micrographs of a untreated Legionella pneumophila ATCC 33153 and b cells of the same strain treated with 1000 µg/mL (40×MIC) penicillin for 5 h, and negatively stained electron micrographs of c untreated L. pneumophila Nottingham N7 and d cells of the same strain treated with 1280 µg/mL (20×MIC) methicillin for 24 h. Arrows show the location of bulge formation. Bar for a 0.2 µm, b 0.4 µm, c 0.5 µm, and d 0.5 µm. Images a and b from [171] and images c and d from [83], all by permission of the Journal of Medical Microbiology (MicroSoc)
Fig. 7
Scanning electron micrographs of a untreated Legionella pneumophila ATCC 33153 and b cells of the same strain treated with 1600 µg/mL (10×MIC) polymyxin B for 5 h, and transmission electron micrographs of c untreated Escherichia coli ATCC 11303 and d cells of the same strain treated with 25 µg/mL (~12.5×MIC) polymyxin B for 30 min. Arrows show the location of some of the blebs. All bars 0.2 µm. Images a and b from [171] by permission of the Journal of Medical Microbiology (MicroSoc), and images c and d from [180] by permission of the Journal of Bacteriology (ASM)
Fig. 8
Transmission electron micrographs of a untreated Staphylococcus aureus ATCC 6538P and b cells of the same strain treated with 0.03 µg/mL (3×MIC) rifampicin for 4 h. Both the septal and peripheral portions of the wall appear thickened following treatment. Bar 1 µm. Images from [155] by permission of Reviews of Infectious Diseases (Oxford University Press)
Fig. 9
Transmission electron micrographs of a untreated Enterobacter cloacae NCTC 1005 and b cells of the same strain treated with 12.5 µg/mL (0.83×MIC) trimethoprim for 4 h. Arrow shows where the layers of the cell envelope appear to have separated. Bar for a 0.5 µm and b 0.25 µm. Images from [195] by permission of the Journal of Medical Microbiology (MicroSoc)
Fig. 10
Transmission electron micrographs of a untreated Enterobacter cloacae NCTC 1005 and b cells of the same strain treated with 12.5 µg/mL (0.83×MIC) trimethoprim for 4 h. Many of the bacterial cells have developed intracellular vacuoles following treatment. Bar 1 µm. Images from [195] by permission of the Journal of Medical Microbiology (MicroSoc)
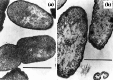
Fig. 11
Transmission electron micrographs of a an untreated clinical isolate of Proteus mirabilis and b cells of the same isolate treated with 0.78 µg/mL (0.5×MIC) ampicillin for 3 h. The number of ribosomes has decreased following treatment. Bar 1 µm. Images from [202] by permission of Proceedings of the Society for Experimental Biology and Medicine (SAGE Publications)
Fig. 12
Transmission electron micrographs of a untreated Staphylococcus aureus ATCC 25923 and b cells of the same strain treated with 0.2 µg/mL (0.5×MIC) quinupristin/dalfopristin for 24 h. Arrows show the location of ghost cells. Magnification for a and b ×37,000. Images from [203] by permission of the Journal of Antimicrobial Chemotherapy (Oxford University Press)
Similar articles
- The bacterial cell wall as a source of antibacterial targets.
Green DW. Green DW. Expert Opin Ther Targets. 2002 Feb;6(1):1-19. doi: 10.1517/14728222.6.1.1. Expert Opin Ther Targets. 2002. PMID: 11901475 Review. - Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics.
Brudzynski K, Sjaarda C. Brudzynski K, et al. PLoS One. 2014 Sep 5;9(9):e106967. doi: 10.1371/journal.pone.0106967. eCollection 2014. PLoS One. 2014. PMID: 25191847 Free PMC article. - Bacterial cell wall assembly: still an attractive antibacterial target.
Bugg TD, Braddick D, Dowson CG, Roper DI. Bugg TD, et al. Trends Biotechnol. 2011 Apr;29(4):167-73. doi: 10.1016/j.tibtech.2010.12.006. Epub 2011 Jan 12. Trends Biotechnol. 2011. PMID: 21232809 Review. - Mechanism of action of antimicrobial agents.
Moellering RC Jr. Moellering RC Jr. Clin Obstet Gynecol. 1979 Jun;22(2):277-83. doi: 10.1097/00003081-197906000-00004. Clin Obstet Gynecol. 1979. PMID: 466873 No abstract available. - New chemical tools to probe cell wall biosynthesis in bacteria.
Gale RT, Brown ED. Gale RT, et al. Curr Opin Microbiol. 2015 Oct;27:69-77. doi: 10.1016/j.mib.2015.07.013. Epub 2015 Aug 25. Curr Opin Microbiol. 2015. PMID: 26291270 Review.
Cited by
- Effect of Photodynamic Therapy on the Pushout Bond Strength of Resin-Based and Calcium Silicate-Based Endodontic Sealers.
Zadsirjan S, Asnaashari M, Yazdani A, Heidari S, Estarami T. Zadsirjan S, et al. J Lasers Med Sci. 2023 Sep 10;14:e33. doi: 10.34172/jlms.2023.33. eCollection 2023. J Lasers Med Sci. 2023. PMID: 38028877 Free PMC article. - Rapid Isolation of Low-Level Carbapenem-Resistant E. coli from Water and Foods Using Glycan-Coated Magnetic Nanoparticles.
Caliskan-Aydogan O, Sharief SA, Alocilja EC. Caliskan-Aydogan O, et al. Biosensors (Basel). 2023 Sep 23;13(10):902. doi: 10.3390/bios13100902. Biosensors (Basel). 2023. PMID: 37887095 Free PMC article. - Monomer and Oligomer Transition of Zinc Phthalocyanine Is Key for Photobleaching in Photodynamic Therapy.
Liu D, Jiang L, Chen J, Chen Z, Yuan C, Lin D, Huang M. Liu D, et al. Molecules. 2023 Jun 8;28(12):4639. doi: 10.3390/molecules28124639. Molecules. 2023. PMID: 37375194 Free PMC article. - Cationic π-Conjugated Polyelectrolyte Shows Antimicrobial Activity by Causing Lipid Loss and Lowering Elastic Modulus of Bacteria.
Zamani E, Johnson TJ, Chatterjee S, Immethun C, Sarella A, Saha R, Dishari SK. Zamani E, et al. ACS Appl Mater Interfaces. 2020 Nov 4;12(44):49346-49361. doi: 10.1021/acsami.0c12038. Epub 2020 Oct 22. ACS Appl Mater Interfaces. 2020. PMID: 33089982 Free PMC article. - SERS-based rapid susceptibility testing of commonly administered antibiotics on clinically important bacteria species directly from blood culture of bacteremia patients.
Han YY, Wang JT, Cheng WC, Chen KL, Chi Y, Teng LJ, Wang JK, Wang YL. Han YY, et al. World J Microbiol Biotechnol. 2023 Aug 17;39(10):282. doi: 10.1007/s11274-023-03717-x. World J Microbiol Biotechnol. 2023. PMID: 37589866 Free PMC article.
References
- McGuire MJ. The chlorine revolution: water disinfection and the fight to save lives. Denver: American Water Works Association; 2013.
- Greenwood D, et al. Chapter 1: Historical introduction. In: Finch RG, et al., editors. Antibiotic and chemotherapy: anti-infective agents and their use in therapy. 9. London: Elsevier Health Sciences; 2010. pp. 2–9.
- Fraise AP, Maillard J-Y, Sattar SA, editors. Russell, Hugo and Ayliffe’s principles and practice of disinfection, preservation, and sterilization. 5. Chichester: Wiley-Blackwell; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical