Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile - PubMed (original) (raw)
Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile
Charisse N Winston et al. Alzheimers Dement (Amst). 2016.
Abstract
Introduction: Levels of Alzheimer's disease (AD)-related proteins in plasma neuronal derived exosomes (NDEs) were quantified to identify biomarkers for prediction and staging of mild cognitive impairment (MCI) and AD.
Methods: Plasma exosomes were extracted, precipitated, and enriched for neuronal source by anti-L1CAM antibody absorption. NDEs were characterized by size (Nanosight) and shape (TEM) and extracted NDE protein biomarkers were quantified by ELISAs. Plasma NDE cargo was injected into normal mice, and results were characterized by immunohistochemistry to determine pathogenic potential.
Results: Plasma NDE levels of P-T181-tau, P-S396-tau, and Aβ1-42 were significantly higher, whereas those of neurogranin (NRGN) and the repressor element 1-silencing transcription factor (REST) were significantly lower in AD and MCI converting to AD (ADC) patients compared to cognitively normal controls (CNC) subjects and stable MCI patients. Mice injected with plasma NDEs from ADC patients displayed increased P-tau (PHF-1 antibody)-positive cells in the CA1 region of the hippocampus compared to plasma NDEs from CNC and stable MCI patients.
Conclusions: Abnormal plasma NDE levels of P-tau, Aβ1-42, NRGN, and REST accurately predict conversion of MCI to AD dementia. Plasma NDEs from demented patients seeded tau aggregation and induced AD-like neuropathology in normal mouse CNS.
Keywords: Alzheimer's disease; Beta amyloid; Biomarker; Exosomes; Mild cognitive impairment; Neuron; Phospho-tau.
Figures
Fig. 1
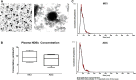
Plasma NDEs derived from stable MCI and ADC patients are similar in size and shape to previously reported exosome preparations. (A) Representative TEM image of plasma NDEs derived from a stable MCI patient (scale bars 350 nm; 100 nm). NTA detects high concentrations of plasma NDEs (B) with similar size distributions (C) that are not significantly different between the two patient populations. (C) Representative NTA plot of averaged size/concentration for plasma NDEs derived from a MCI and ADC patient (n = 4/group). Abbreviations: NDE, neuronal derived exosome; MCI, mild cognitive impairment; ADC, MCI converting to AD; TEM, transmission electron microscopy; NTA, nanoparticle tracking analysis.
Fig. 2
Human plasma NDE levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST delineate stages of AD. (A) Plasma NDE levels of CD81 were not statistically different among the four clinical cohorts as measured by ELISA. Significantly different levels of (B) Aβ1–42, (C) P-T181-tau, (D) P-S396-tau, (E) NRGN, and (F) REST were detected by ELISAs in the plasmas of CNC (n = 10), stable MCI (n = 20), ADC (n = 20), and AD (n = 10) patients. Plasma NDE levels of P-T181-tau, P-S396-tau, and Aβ1–42 were significantly higher, whereas those of NRGN and REST were significantly lower for ADC and AD patients than CNC subjects and stable MCI patients. The horizontal line in each cluster depicts the mean for that set. * = P < .05, ** = P < .01, *** = P < .0001 versus CNC; ## = P < .01, ### = P < .0001 versus MCI. Abbreviations: NDE, neuronal derived exosome; CNC, cognitively normal controls; MCI, mild cognitive impairment; ADC, MCI converting to AD.
Fig. 3
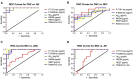
ROC curve analyses assess diagnostic accuracy for plasma NDEs levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST. ROC curves based on the plasma NDE levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST for (A) CNC subjects versus AD patients, (B) CNC subjects versus stable MCI patients, (C) stable MCI versus ADC patients, and (D) stable MCI versus AD patients. Abbreviations: ROC, receiver operating curve; NDE, neuronal derived exosome; CNC, cognitively normal control; MCI, mild cognitive impairment; ADC, MCI converting to AD.
Fig. 4
Plasma NDEs from stable MCI and AD patients seed tau aggregation in the brains of normal mice. Representative photomicrographs of hippocampal sections obtained from wild-type, C57/Bl6 mice that were injected with plasma NDEs from control, stable MCI, and ADC patients. At 1-month post-injection, mice injected with plasma NDEs from stable MCI patients display modest enhancement of PHF-1 immunoreactivity in the CA1 region of the hippocampus, whereas mice injected with plasma NDEs from ADC patients display a significant increase in PHF-1 immunoreactivity in the CA1. Extensive cellular staining as well as staining within the dendritic processes can be observed in these mice. Mice-injected NDEs from control plasma displayed no PHF-1 immunoreactivity (n = 6/group; scale bars 250 μm; 25 μm). Abbreviations: NDE, neuronal derived exosome; MCI, mild cognitive impairment; ADC, MCI converting to AD.
Similar articles
- Growth Hormone-Releasing Hormone Modulation of Neuronal Exosome Biomarkers in Mild Cognitive Impairment.
Winston CN, Goetzl EJ, Baker LD, Vitiello MV, Rissman RA. Winston CN, et al. J Alzheimers Dis. 2018;66(3):971-981. doi: 10.3233/JAD-180302. J Alzheimers Dis. 2018. PMID: 30372675 Free PMC article. Clinical Trial. - Clinical significance of the cognition-related pathogenic proteins in plasma neuronal-derived exosomes among normal cognitive adults over 45 years old with olfactory dysfunction.
Chen Z, Chang F, Yao L, Yuan F, Hong J, Wu D, Wei Y. Chen Z, et al. Eur Arch Otorhinolaryngol. 2022 Jul;279(7):3467-3476. doi: 10.1007/s00405-021-07143-3. Epub 2021 Oct 24. Eur Arch Otorhinolaryngol. 2022. PMID: 34693486 - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review. - Increased prediction value of biomarker combinations for the conversion of mild cognitive impairment to Alzheimer's dementia.
Zhao A, Li Y, Yan Y, Qiu Y, Li B, Xu W, Wang Y, Liu J, Deng Y. Zhao A, et al. Transl Neurodegener. 2020 Aug 3;9(1):30. doi: 10.1186/s40035-020-00210-5. Transl Neurodegener. 2020. PMID: 32741361 Free PMC article. - Emerging blood exosome-based biomarkers for preclinical and clinical Alzheimer's disease: a meta-analysis and systematic review.
Liu WL, Lin HW, Lin MR, Yu Y, Liu HH, Dai YL, Chen LW, Jia WW, He XJ, Li XL, Zhu JF, Xue XH, Tao J, Chen LD. Liu WL, et al. Neural Regen Res. 2022 Nov;17(11):2381-2390. doi: 10.4103/1673-5374.335832. Neural Regen Res. 2022. PMID: 35535875 Free PMC article. Review.
Cited by
- Functional Nanomaterials for the Diagnosis of Alzheimer's Disease: Recent Progress and Future Perspectives.
Al Abdullah S, Najm L, Ladouceur L, Ebrahimi F, Shakeri A, Al-Jabouri N, Didar TF, Dellinger K. Al Abdullah S, et al. Adv Funct Mater. 2023 Sep 12;33(37):2302673. doi: 10.1002/adfm.202302673. Epub 2023 May 24. Adv Funct Mater. 2023. PMID: 39309539 Free PMC article. - The Cell Biology of Tau Secretion.
Merezhko M, Uronen RL, Huttunen HJ. Merezhko M, et al. Front Mol Neurosci. 2020 Sep 23;13:569818. doi: 10.3389/fnmol.2020.569818. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33071756 Free PMC article. Review. - Extracellular Vesicles Isolated from Familial Alzheimer's Disease Neuronal Cultures Induce Aberrant Tau Phosphorylation in the Wild-Type Mouse Brain.
Aulston B, Liu Q, Mante M, Florio J, Rissman RA, Yuan SH. Aulston B, et al. J Alzheimers Dis. 2019;72(2):575-585. doi: 10.3233/JAD-190656. J Alzheimers Dis. 2019. PMID: 31594233 Free PMC article. - Tau-Reactive Endogenous Antibodies: Origin, Functionality, and Implications for the Pathophysiology of Alzheimer's Disease.
Hromadkova L, Ovsepian SV. Hromadkova L, et al. J Immunol Res. 2019 Aug 6;2019:7406810. doi: 10.1155/2019/7406810. eCollection 2019. J Immunol Res. 2019. PMID: 31687413 Free PMC article. Review. - L1CAM-associated extracellular vesicles: A systematic review of nomenclature, sources, separation, and characterization.
Gomes DE, Witwer KW. Gomes DE, et al. J Extracell Biol. 2022 Mar;1(3):e35. doi: 10.1002/jex2.35. Epub 2022 Apr 5. J Extracell Biol. 2022. PMID: 35492832 Free PMC article.
References
- Ward A., Arrighi H.M., Michels S., Cedarbaum J.M. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. - PubMed
- Grundman M., Petersen R.C., Ferris S.H., Thomas R.G., Aisen P.S., Bennett D.A. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. - PubMed
- van Rossum I.A., Vos S., Handels R., Visser P.J. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J Alzheimers Dis. 2010;20:881–891. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous