Deletion of DXZ4 on the human inactive X chromosome alters higher-order genome architecture - PubMed (original) (raw)
. 2016 Aug 2;113(31):E4504-12.
doi: 10.1073/pnas.1609643113. Epub 2016 Jul 18.
Miriam H Huntley 2, Olga Dudchenko 3, Elena K Stamenova 4, Neva C Durand 5, Zhuo Sun 1, Su-Chen Huang 5, Adrian L Sanborn 6, Ido Machol 5, Muhammad Shamim 5, Andrew P Seberg 1, Eric S Lander 7, Brian P Chadwick 8, Erez Lieberman Aiden 9
Affiliations
- PMID: 27432957
- PMCID: PMC4978254
- DOI: 10.1073/pnas.1609643113
Deletion of DXZ4 on the human inactive X chromosome alters higher-order genome architecture
Emily M Darrow et al. Proc Natl Acad Sci U S A. 2016.
Abstract
During interphase, the inactive X chromosome (Xi) is largely transcriptionally silent and adopts an unusual 3D configuration known as the "Barr body." Despite the importance of X chromosome inactivation, little is known about this 3D conformation. We recently showed that in humans the Xi chromosome exhibits three structural features, two of which are not shared by other chromosomes. First, like the chromosomes of many species, Xi forms compartments. Second, Xi is partitioned into two huge intervals, called "superdomains," such that pairs of loci in the same superdomain tend to colocalize. The boundary between the superdomains lies near DXZ4, a macrosatellite repeat whose Xi allele extensively binds the protein CCCTC-binding factor. Third, Xi exhibits extremely large loops, up to 77 megabases long, called "superloops." DXZ4 lies at the anchor of several superloops. Here, we combine 3D mapping, microscopy, and genome editing to study the structure of Xi, focusing on the role of DXZ4 We show that superloops and superdomains are conserved across eutherian mammals. By analyzing ligation events involving three or more loci, we demonstrate that DXZ4 and other superloop anchors tend to colocate simultaneously. Finally, we show that deleting DXZ4 on Xi leads to the disappearance of superdomains and superloops, changes in compartmentalization patterns, and changes in the distribution of chromatin marks. Thus, DXZ4 is essential for proper Xi packaging.
Keywords: CTCF; Hi‐C; X chromosome inactivation; genome engineering; inactive X chromosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
The Xi chromosome superstructure is conserved across human, rhesus macaque, and mouse. (A) Superdomains on the Xi chromosome are conserved across human, rhesus macaque, and mouse. The boundary of the superdomains lies at DXZ4 and its orthologs. In diploid Hi-C maps of mouse, the superdomain is seen only on the Xi chromosome. (Resolution: 100 kb.) For all contact maps, the color scale of each map goes from 0 (white) to red, whose value is given by the red square in each map. The chromosome icons are colored gray to indicate unphased maps of the X chromosome, in which data from both the Xi and Xa chromosomes are superimposed; they are colored red to indicate diploid Xi-only maps or green to indicate diploid Xa-only maps. The phased SNP calls used to generate homolog-specific maps are outlined in SI Appendix, Supplementary Materials and Methods. (B) A superloop forms between DXZ4 and FIRRE in human. Superloops are present at orthologous positions in rhesus macaque and mouse. In diploid Hi-C maps of mouse, the superloop is only seen on the Xi chromosome. (Resolution: 50 kb.)
Fig. 2.
Concatemers produced by proximity ligation indicate simultaneous colocation of three or more loci. (A) In COLA, concatemers spanning three or more fragments are created by cutting chromatin using CviJI, followed by DNA–DNA proximity ligation in intact nuclei. (B) Contact triples visualized as a 3D contact tensor. Broad patterns of contacts can be revealed by slicing the tensor and examining the results at low resolution. (C) A planar cut of the contact tensor enables the examination of all triples containing DXZ4. Enlargement: Enrichment is seen in the plane at x75(–DXZ4)–FIRRE. (Resolution: 800 kb.) (D) A different cut makes it possible to study triples in the vicinity of ICCE. Superloop triples (_ICCE_–)DXZ4–FIRRE and (ICCE–)x75–FIRRE are highlighted. (Resolution: 2 Mb.)
Fig. 3.
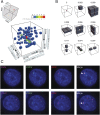
The ICCE_–_DXZ4 and DXZ4_–_FIRRE superloops tend to occur simultaneously, forming a hub on the Xi chromosome. (A) Examination of a small region from the 3D contact tensor of the X chromosome, centered on ICCE, DXZ4, and FIRRE, reveals a peak relative to the local neighborhood. Five contacts are seen in the (300 kb)3 voxel (i.e., 3D pixel) associated with simultaneous colocation of all three loci. There are more than 2,000 other (300 kb)3 voxels in the region shown; the number of contacts in each is indicated by the color. Of these voxels, five contain two contacts each, 74 contain one contact each, and more than 2,000 voxels contain no contacts. Note that because of the fixed bin width, the voxel size presented in this figure, (300 kb)3, is slightly larger than (and has slightly more contacts than) the exact volume defined by ICCE_–_DXZ4_–_FIRRE boundaries, which was used for the analyses in the main text. (B) The average frequency of contact in various local neighborhoods surrounding the ICCE_–_DXZ4_–_FIRRE peak. The peak is strongly enriched with respect to every model. (C) Representative examples of direct-labeled three-color DNA FISH in GM12878, a female cell line, showing collocation of ICCE_–_DXZ4_–_FIRRE. FISH signals overlay DAPI (blue) and are merged in the panel at the far right. The white arrowhead indicates a three-way overlap on one X chromosome.
Fig. 4.
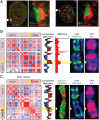
Deletion of DX4 disrupts compartmentalization, distribution of histone marks, and replication timing. (A) Immunofluorescence showing the distribution of H3K9me3 (green, indirect immunofluorescence) and H3K27me3 (red, direct immunofluorescence) in wild-type RPE1 cells at interphase. White arrowheads indicate the location of the Xi chromosome that is expanded in the panels to the right, showing the corresponding nuclei. (B) A correlation matrix derived from the contact map of the wild-type RPE1 Xi chromosome reveals two distinct long-range contact patterns, indicating two subcompartments (first column, numbered left to right). These patterns are reflected in its principal eigenvector, which is shown to the right of the matrix (second column). The color of the eigenvector indicates its sign and thus the long-range pattern exhibited by the corresponding locus (Resolution: 500 kb.) One of these subcompartments correlates well with H3K27me3 ChIP-Seq in RPE1 (third column), as well as with a representative metaphase Xi chromosome showing the distribution of H3K27me3 (green, indirect immunofluorescence) merged with DAPI (blue) in RPE1 (fourth column), and with a representative metaphase Xi chromosome showing the pattern of EdU incorporation (red) merged with DAPI (blue) (fifth column). The yellow arrowheads indicate the H3K27me3 band between x100 and DXZ4 that replicates earlier in S-phase. (C) A correlation matrix derived from the contact map of the RPE1-ΔDXZ4i Xi chromosome (first column); its principal eigenvector (second column); a representative RPE1-ΔDXZ4i metaphase Xi chromosome showing the distribution of H3K9me3 (green, indirect immunofluorescence) and H3K27me3 (red, direct immunofluorescence) (third column); a representative metaphase Xi chromosome showing the distribution of H3K27me3 (green, indirect immunofluorescence) merged with DAPI (blue) (fourth column); and a representative metaphase Xi chromosome showing the pattern of EdU incorporation (red) merged with DAPI (blue) (fifth column). The compartment interval between x100 and DX74 is compromised, and corresponding changes are seen in metaphase histone mark distribution and in replication timing.
Fig. 5.
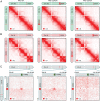
Deletion of DXZ4 eliminates the Xi chromosome superstructure. (A) Maps of the Xa chromosome in RPE1 (Left), RPE1-ΔDXZ4a (Center), and RPE1-ΔDXZ4i (Right) cells. Compartmentalization is seen. Superstructure is absent. The chromosome icons are colored gray to indicate unphased maps of the X chromosome, in which data from both Xi and Xa chromosomes are superimposed; they are colored red to indicate diploid Xi-only maps; and they are colored green to indicate diploid Xa-only maps. The phased SNP calls used to generate homolog-specific maps are outlined in the SI Appendix. (B) Maps of the Xi chromosome in RPE1 (Left), RPE1-ΔDXZ4a (Center), and RPE1-ΔDXZ4i (Right) cells exhibit compartmentalization with unusually long compartment intervals. Superdomains are present in wild-type RPE1 cells and remain after DXZ4 is deleted on the Xa chromosome but not after DXZ4 is deleted on the Xi chromosome. (Resolution: 500 kb.) (C) The DXZ4_–_FIRRE superloop is present in wild-type RPE1 (Left) and in RPE1-ΔDXZ4a (Center) cells but disappears in RPE1-ΔDXZ4i cells (Right). (Resolution: 50 kb.)
Fig. 6.
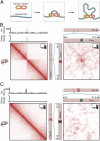
(A) In our physical model of loop formation by extrusion (26), an extrusion complex comprising two DNA-binding subunits is loaded onto chromatin. The subunits form a loop by sliding in opposite directions. When they arrive at an anchor site, they have a probability of binding and thus halting the extrusion process. (B) We generated an ensemble of Patski Xi chromosome configurations using the extrusion model, with anchor-binding probabilities drawn from Ctcf ChIP-Seq data in Patski. We calculated a contact map from this ensemble. (Left) A superdomain boundary is formed at Dxz4. (Right) A superloop forms between Dxz4 and Firre. The icon in the upper right corner of each map indicates that these maps were generated in silico, using physical simulations, rather than via experiment. (C) Simulating the deletion of Dxz4 leads to the disappearance of the superdomain (Left) and superloop (Right).
Similar articles
- Characterization of the ICCE Repeat in Mammals Reveals an Evolutionary Relationship with the DXZ4 Macrosatellite through Conserved CTCF Binding Motifs.
Westervelt N, Chadwick BP. Westervelt N, et al. Genome Biol Evol. 2018 Sep 1;10(9):2190-2204. doi: 10.1093/gbe/evy176. Genome Biol Evol. 2018. PMID: 30102341 Free PMC article. - The macrosatellite DXZ4 mediates CTCF-dependent long-range intrachromosomal interactions on the human inactive X chromosome.
Horakova AH, Moseley SC, McLaughlin CR, Tremblay DC, Chadwick BP. Horakova AH, et al. Hum Mol Genet. 2012 Oct 15;21(20):4367-77. doi: 10.1093/hmg/dds270. Epub 2012 Jul 12. Hum Mol Genet. 2012. PMID: 22791747 Free PMC article. - Orientation-dependent Dxz4 contacts shape the 3D structure of the inactive X chromosome.
Bonora G, Deng X, Fang H, Ramani V, Qiu R, Berletch JB, Filippova GN, Duan Z, Shendure J, Noble WS, Disteche CM. Bonora G, et al. Nat Commun. 2018 Apr 13;9(1):1445. doi: 10.1038/s41467-018-03694-y. Nat Commun. 2018. PMID: 29654302 Free PMC article. - Structural aspects of the inactive X chromosome.
Bonora G, Disteche CM. Bonora G, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Nov 5;372(1733):20160357. doi: 10.1098/rstb.2016.0357. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28947656 Free PMC article. Review. - Multifaceted role of CTCF in X-chromosome inactivation.
Bammidi LS, Gayen S. Bammidi LS, et al. Chromosoma. 2024 Oct;133(4):217-231. doi: 10.1007/s00412-024-00826-w. Epub 2024 Oct 21. Chromosoma. 2024. PMID: 39433641 Review.
Cited by
- Extracting multi-way chromatin contacts from Hi-C data.
Liu L, Zhang B, Hyeon C. Liu L, et al. PLoS Comput Biol. 2021 Dec 6;17(12):e1009669. doi: 10.1371/journal.pcbi.1009669. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34871311 Free PMC article. - SCL: a lattice-based approach to infer 3D chromosome structures from single-cell Hi-C data.
Zhu H, Wang Z. Zhu H, et al. Bioinformatics. 2019 Oct 15;35(20):3981-3988. doi: 10.1093/bioinformatics/btz181. Bioinformatics. 2019. PMID: 30865261 Free PMC article. - Local rewiring of genome-nuclear lamina interactions by transcription.
Brueckner L, Zhao PA, van Schaik T, Leemans C, Sima J, Peric-Hupkes D, Gilbert DM, van Steensel B. Brueckner L, et al. EMBO J. 2020 Mar 16;39(6):e103159. doi: 10.15252/embj.2019103159. Epub 2020 Feb 21. EMBO J. 2020. PMID: 32080885 Free PMC article. - Spatial promoter-enhancer hubs in cancer: organization, regulation, and function.
Zhao J, Faryabi RB. Zhao J, et al. Trends Cancer. 2023 Dec;9(12):1069-1084. doi: 10.1016/j.trecan.2023.07.017. Epub 2023 Aug 19. Trends Cancer. 2023. PMID: 37599153 Free PMC article. Review. - Three-dimensional organization and dynamics of the genome.
Szalaj P, Plewczynski D. Szalaj P, et al. Cell Biol Toxicol. 2018 Oct;34(5):381-404. doi: 10.1007/s10565-018-9428-y. Epub 2018 Mar 22. Cell Biol Toxicol. 2018. PMID: 29568981 Free PMC article. Review.
References
- Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. - PubMed
- Brockdorff N, et al. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71(3):515–526. - PubMed
- Brown CJ, et al. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71(3):527–542. - PubMed
- Dixon-McDougall T, Brown C. The making of a Barr body: The mosaic of factors that eXIST on the mammalian inactive X chromosome. Biochem Cell Biol. 2016;94(1):56–70. - PubMed
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7031):400–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL130010/HL/NHLBI NIH HHS/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- DP2 OD008540/OD/NIH HHS/United States
- P50 HG006193/HG/NHGRI NIH HHS/United States
- R01 GM073120/GM/NIGMS NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases