Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans - PubMed (original) (raw)
. 2016 Aug 2;113(31):8759-64.
doi: 10.1073/pnas.1601442113. Epub 2016 Jul 18.
Collaborators, Affiliations
- PMID: 27432961
- PMCID: PMC4978273
- DOI: 10.1073/pnas.1601442113
Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans
Vivian S Lee et al. Proc Natl Acad Sci U S A. 2016.
Abstract
Thoracic aortic aneurysms and dissections (TAAD) represent a substantial cause of morbidity and mortality worldwide. Many individuals presenting with an inherited form of TAAD do not have causal mutations in the set of genes known to underlie disease. Using whole-genome sequencing in two first cousins with TAAD, we identified a missense mutation in the lysyl oxidase (LOX) gene (c.893T > G encoding p.Met298Arg) that cosegregated with disease in the family. Using clustered regularly interspaced short palindromic repeats (CRISPR)/clustered regularly interspaced short palindromic repeats-associated protein-9 nuclease (Cas9) genome engineering tools, we introduced the human mutation into the homologous position in the mouse genome, creating mice that were heterozygous and homozygous for the human allele. Mutant mice that were heterozygous for the human allele displayed disorganized ultrastructural properties of the aortic wall characterized by fragmented elastic lamellae, whereas mice homozygous for the human allele died shortly after parturition from ascending aortic aneurysm and spontaneous hemorrhage. These data suggest that a missense mutation in LOX is associated with aortic disease in humans, likely through insufficient cross-linking of elastin and collagen in the aortic wall. Mutation carriers may be predisposed to vascular diseases because of weakened vessel walls under stress conditions. LOX sequencing for clinical TAAD may identify additional mutation carriers in the future. Additional studies using our mouse model of LOX-associated TAAD have the potential to clarify the mechanism of disease and identify novel therapeutics specific to this genetic cause.
Keywords: CRISPR/Cas9; aortic dissection; genetics; lysyl oxidase; whole-genome sequencing.
Conflict of interest statement
Conflict of interest statement: N.O.S. reports research support from AstraZenica and has served as a consultant to Aegerion Pharmaceuticals, both outside of the scope of this work. The other authors have no disclosures to report.
Figures
Fig. 1.
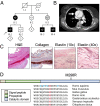
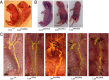
FTAAD associated with LOX mutation. (A) The family’s pedigree shows autosomal dominant inheritance of FTAAD. Black symbols indicate affected individuals with arterial dissection or aneurysm. Gray symbols indicate individuals affected with arterial tortuosity. White symbols indicate unaffected individuals; − indicates presence of the mutation. (B) Contrast-enhanced axial computed tomography image from individual II-1 showing ascending aortic aneurysm (arrow) with dissection and an intimal flap separating the false and true lumens. (C) Histologic evaluation of aortic tissue resected from individual II-1. H&E staining shows abnormal architecture and a dissection tear. Masson’s trichrome staining for collagen (blue) reveals disorganization of the collagen fibers and disruption of the medial architecture. Verhoeff–van Gieson staining for elastin (dark-purple fibers) illustrates disarray and fragmentation of elastic fibers. (D) The location of the M298R missense mutation is depicted in relation to the domains of the LOX protein. Methionine at position 298 is highly conserved as shown by the homologous protein sequences from multiple organisms shown below. Histidine positions that are essential for copper binding and/or LOX catalytic activity (14) are highlighted in blue.
Fig. S1.
Identification of a heterozygous p.Met298Arg LOX mutation in affected individuals. Sequence chromatograms of genomic PCR products are shown. The sequence of the normal allele from the unaffected individual II-2 is shown in Upper. The sequence of the mutant c.893T > G (p.Met298Arg) LOX allele from the affected individual II-1 is shown in Lower.
Fig. 2.
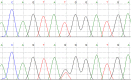
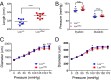
Lox +/Mut animals have altered aortic wall dimensions and normal blood pressure. (A) The length of the ascending aorta from the heart to the brachiocephalic artery is significantly longer in 3-mo-old Lox +/Mut animals compared with Lox+/+ littermate controls. ****P = 0.0005. (B) Blood pressure measurements using an arterial catheter found no difference in systolic (P = 0.15) or diastolic (P = 0.87) blood pressure between Lox +/+ and Lox +/Mut mice. n.s., not significant. Pressure–diameter responses collected for (C) the left common carotid artery and (D) ascending aorta show that both vessels in Lox +/Mut animals are slightly stiffer than controls at high pressure but are not different in the physiological pressure range. Data are from n = 9 Lox _+/_Mut and n = 7 Lox+/+ animals. *P = 0.02; **P = 0.001; ***P = 0.0002.
Fig. S2.
Sequence verification of mouse genotypes. Sequence chromatograms are shown for mice that are (A) Lox +/+, (B) Lox +/Mut, and (C) Lox Mut/Mut.
Fig. 3.
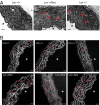
Lox +/Mut animals have altered ultrastructural properties of the aortic wall. (A) EM of the ascending aorta from Lox +/+, Lox +/Mut, and Lox +/− mice. The aortic walls of Lox +/+ animals showed smooth and continuous elastic lamellae (white arrowhead), whereas aortas from both Lox +/Mut, and Lox +/− mice were found to be thicker with fragmented (red arrowheads) and disorganized elastic lamellae. (Scale bar: 10 µm.) *Aortic lumen. (B) Autofluorescence of elastin in aortic tissue showed that this was not an isolated finding and found that Lox +/Mut animals had a significantly higher density of elastic lamellae breaks (red arrowheads) compared with Lox +/+ mice (P = 0.0006). (Scale bar: 20 µm.) *Aortic lumen.
Fig. 4.
Lox Mut/Mut exhibit normal size, kyphosis, hemorrhages, and arterial tortuosity, and they die perinatally of aortic aneurysm/dissection. (A) Lox Mut/Mut animals were born in the expected Mendelian numbers but died within a few hours of birth. Body size of the mutant animals was comparable with WT and heterozygous littermates. Some Lox Mut/Mut animals had severe kyphosis (arrowhead in A), and cranial, thoracic, and abdominal hemorrhages (arrows in B) were common. Arterial architecture visualized by yellow latex injection into the left ventricle showed that Lox Mut/Mut animals have highly tortuous vessels together with ascending and abdominal aortic aneurysms (arrows in C). Vessel tortuosity or aneurysms were not observed in Lox +/+ or Lox +/Mut littermates. Blood clots around blood vessels (arrowheads in C) indicated that aneurysmal rupture was a frequent occurrence in Lox Mut/Mut mice. (Scale bar: 1 mm.)
Fig. S3.
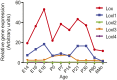
Lox is the predominant Lox family member expressed in developing mouse aorta. Data from our previous microarray study (44) were used to ascertain Lox family member expression levels in developing mouse aorta. The data showed that Lox was the LOX gene family member with highest expression levels at all developmental time points. E, embryonic day; P, postnatal day.
Fig. 5.
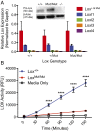
Mutant Lox is expressed and synthesized but lacks enzymatic activity. (A) Quantitative RT-PCR analysis of mRNA from the aortas of Lox +/+, Lox +/Mut, and Lox Mut/Mut P0 animals showed normal expression levels of all Lox family members in all three genotypes (note that the Lox primer/probe recognizes both Lox + and Lox Mut alleles). Two-way ANOVA P = 0.63 for Lox +/+ vs. Lox +/Mut; P = 0.99 for Lox +/+ vs. Lox Mut/Mut; and P = 0.63 for Lox +/Mut vs. Lox Mut/Mut. (Inset) Lox protein immunoblotting confirmed that the mutant Lox protein is produced by Lox Mut/Mut cells at levels similar to the WT (Lox +/+) protein. The absence of a band in extracts of Lox −/− cells confirms the specificity of the Lox antibody. (B) Lox activity in the presence and absence of β-aminopropionitrile (BAPN) was assayed in conditioned culture medium from Lox +/+ and Lox Mut/Mut MEFs at 30-min time intervals. Medium incubated without cells served as the baseline control. MEFs cultured from two Lox +/+ and three Lox Mut/Mut embryos were tested in duplicates for n = 4 and n = 6, respectively, and plotted according to genotype at each time point (mean ± SEM). Lox activity, which is the difference between activity with and without BAPN, is expressed in relative fluorescent units (RFUs). ****P < 0.0001.
Fig. S4.
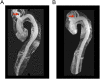
Representative images taken from the 3D reconstructions magnetic resonance angiograms. (A) Individual II-3 ,who inherited the LOX p.M298R mutation, was found to have severely tortuous aortic branch vessels, including the common carotid arteries (arrow). (B) Individual II-2, who did not inherit the LOX p.M298R mutation, was found to have normal carotid arteries without tortuosity (arrow).
Similar articles
- Novel LOX Variants in Five Families with Aortic/Arterial Aneurysm and Dissection with Variable Connective Tissue Findings.
Van Gucht I, Krebsova A, Diness BR, Laga S, Adlam D, Kempers M, Samani NJ, Webb TR, Baranowska AA, Van Den Heuvel L, Perik M, Luyckx I, Peeters N, Votypka P, Macek M, Meester J, Van Laer L, Verstraeten A, Loeys BL. Van Gucht I, et al. Int J Mol Sci. 2021 Jul 1;22(13):7111. doi: 10.3390/ijms22137111. Int J Mol Sci. 2021. PMID: 34281165 Free PMC article. - Mechanical behavior and matrisome gene expression in the aneurysm-prone thoracic aorta of newborn lysyl oxidase knockout mice.
Staiculescu MC, Kim J, Mecham RP, Wagenseil JE. Staiculescu MC, et al. Am J Physiol Heart Circ Physiol. 2017 Aug 1;313(2):H446-H456. doi: 10.1152/ajpheart.00712.2016. Epub 2017 May 26. Am J Physiol Heart Circ Physiol. 2017. PMID: 28550176 Free PMC article. - LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections.
Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ; University of Washington Center for Mendelian Genomics; Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM. Guo DC, et al. Circ Res. 2016 Mar 18;118(6):928-34. doi: 10.1161/CIRCRESAHA.115.307130. Epub 2016 Jan 12. Circ Res. 2016. PMID: 26838787 Free PMC article. - Successes and challenges of using whole exome sequencing to identify novel genes underlying an inherited predisposition for thoracic aortic aneurysms and acute aortic dissections.
Milewicz DM, Regalado ES, Shendure J, Nickerson DA, Guo DC. Milewicz DM, et al. Trends Cardiovasc Med. 2014 Feb;24(2):53-60. doi: 10.1016/j.tcm.2013.06.004. Epub 2013 Aug 15. Trends Cardiovasc Med. 2014. PMID: 23953976 Free PMC article. Review. - The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective.
Ostberg NP, Zafar MA, Ziganshin BA, Elefteriades JA. Ostberg NP, et al. Biomolecules. 2020 Jan 24;10(2):182. doi: 10.3390/biom10020182. Biomolecules. 2020. PMID: 31991693 Free PMC article. Review.
Cited by
- Elastic fibers and biomechanics of the aorta: Insights from mouse studies.
Yanagisawa H, Wagenseil J. Yanagisawa H, et al. Matrix Biol. 2020 Jan;85-86:160-172. doi: 10.1016/j.matbio.2019.03.001. Epub 2019 Mar 15. Matrix Biol. 2020. PMID: 30880160 Free PMC article. Review. - Potential Molecular Pathways Related to Pulmonary Artery Aneurysm Development: Lessons to Learn from the Aorta.
Nuche J, Palomino-Doza J, Ynsaurriaga FA, Delgado JF, Ibáñez B, Oliver E, Escribano Subías P. Nuche J, et al. Int J Mol Sci. 2020 Apr 4;21(7):2509. doi: 10.3390/ijms21072509. Int J Mol Sci. 2020. PMID: 32260370 Free PMC article. Review. - Novel LOX Variants in Five Families with Aortic/Arterial Aneurysm and Dissection with Variable Connective Tissue Findings.
Van Gucht I, Krebsova A, Diness BR, Laga S, Adlam D, Kempers M, Samani NJ, Webb TR, Baranowska AA, Van Den Heuvel L, Perik M, Luyckx I, Peeters N, Votypka P, Macek M, Meester J, Van Laer L, Verstraeten A, Loeys BL. Van Gucht I, et al. Int J Mol Sci. 2021 Jul 1;22(13):7111. doi: 10.3390/ijms22137111. Int J Mol Sci. 2021. PMID: 34281165 Free PMC article. - Molecular phenotyping and functional assessment of smooth muscle-like cells with pathogenic variants in aneurysm genes ACTA2, MYH11, SMAD3 and FBN1.
Burger J, Bogunovic N, de Wagenaar NP, Liu H, van Vliet N, IJpma A, Maugeri A, Micha D, Verhagen HJM, Ten Hagen TLM, Majoor-Krakauer D, van der Pluijm I, Essers J, Yeung KK. Burger J, et al. Hum Mol Genet. 2021 Nov 16;30(23):2286-2299. doi: 10.1093/hmg/ddab190. Hum Mol Genet. 2021. PMID: 34244757 Free PMC article. - ADAMTSL2 gene variant in patients with features of autosomal dominant connective tissue disorders.
Steinle J, Hossain WA, Lovell S, Veatch OJ, Butler MG. Steinle J, et al. Am J Med Genet A. 2021 Mar;185(3):743-752. doi: 10.1002/ajmg.a.62030. Epub 2020 Dec 27. Am J Med Genet A. 2021. PMID: 33369194 Free PMC article.
References
- Guo DC, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39(12):1488–1493. - PubMed
- Lee B, et al. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991;352(6333):330–334. - PubMed
- Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–281. - PubMed
- Loeys BL, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355(8):788–798. - PubMed
- Tsipouras P, et al. Ehlers-Danlos syndrome type IV: Cosegregation of the phenotype to a COL3A1 allele of type III procollagen. Hum Genet. 1986;74(1):41–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL105314/HL/NHLBI NIH HHS/United States
- K08 HL114642/HL/NHLBI NIH HHS/United States
- R00 HG007229/HG/NHGRI NIH HHS/United States
- T32 HL125241/HL/NHLBI NIH HHS/United States
- K12 HD076224/HD/NICHD NIH HHS/United States
- R01 HL131961/HL/NHLBI NIH HHS/United States
- R01 HL053325/HL/NHLBI NIH HHS/United States
- T32 EB018266/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials